Journal scan: A review of 12 recent papers of immediate clinical significance, harvested from major international journals
From the desk of the Editor-in-chief
A 9-Year-Old Child with a Large Thoracic Mass
(1). Chenghao Chen, JAMA. Published online October 3, 2024. doi:10.1001/jama.2024.16482
Case
A previously healthy 9-year-old boy presented to a primary care clinic with a 10-day history of a nonproductive cough without fever, wheeze, chest pain, or abdominal pain. His white blood cell count and C-reactive protein level were normal. A chest radiograph revealed an opacity in the lower right lobe. The patient underwent non-contrast computed tomography (CT) of the chest, which revealed a right anterior mediastinal mass. He was referred the same day to the surgery clinic, where his blood pressure was 113/81 mm Hg; heart rate, 98/min; respiratory rate, 20/min; and oxygen saturation, 98% while breathing room air. On physical examination, his chest wall appeared normal. Lung examination revealed diminished breath sounds in the right lower lung field. Levels of α-fetoprotein, human chorionic gonadotropin, and neuron-specific enolase were normal. A contrast-enhanced chest CT scan revealed a 10.4 × 7.8 × 7.8–cm mass in the right anterior mediastinum with a radiodensity of −121 Hounsfield units, consistent with fat (Figure).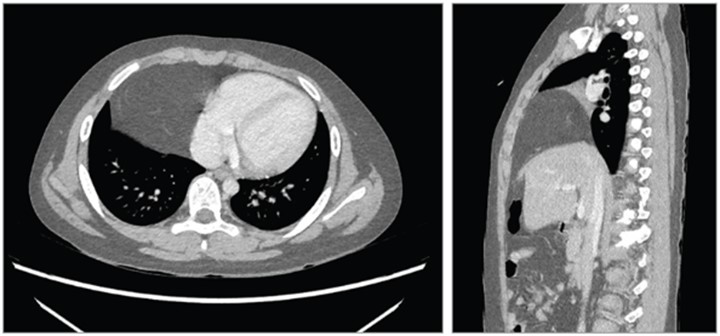
Computed tomography scan of the patient’s chest. Left, Transverse view showing a 10.4 × 7.8 × 7.8–cm mass (−121 Hounsfield units) in the right lower thoracic cavity adjacent to the heart, which was displaced to the left. Right, Sagittal view showing an anterior mediastinal mass in the right lower thoracic cavity and a diaphragm that appeared discontinuous.
What Would You Do Next?
- Arrange for upper gastrointestinal tract endoscopy?
- Perform a percutaneous biopsy of the mass?
- Prescribe intravenous cefuroxime (20 mg/kg/d for 7 days)?
- Schedule laparoscopic surgery?
Diagnosis: Morgagni hernia
The key to the correct diagnosis is recognition that presence of a fat-density mass in the thoracic cavity along with signs of a discontinuous diaphragm are characteristic of a congenital diaphragmatic hernia (CDH). Upper gastrointestinal tract endoscopy (choice A) is incorrect because the anterior mediastinal mass did not appear related to the esophagus or stomach. Percutaneous biopsy (choice B) is not recommended because it could penetrate herniated bowel and cause bleeding if the gastro-omental blood vessels are punctured. Prescribing antibiotics (choice C) is not indicated because the patient had no clinical signs of infection.
Discussion
Congenital diaphragmatic hernia is a developmental defect of the diaphragm, with an incidence rate of approximately 2.3 per 10 000 live births.1 Mortality estimates range from 10% to 90%, based on factors such as the severity of the diaphragmatic defect, presence of other congenital malformations, and access to specialized care.1
The 3 types of CDH are Bochdalek hernias, hiatal hernias, and Morgagni hernias. Bochdalek hernias account for 95% of CDHs and are caused by absence of an intact posterolateral diaphragm; most (85%) occur on the left side.2 Hiatal hernias are caused by developmental abnormality of the esophageal hiatus and lead to herniation of the gastroesophageal junction, stomach, or intestine through the esophageal hiatus. Morgagni hernias, which account for 2% to 4% of CDHs, are caused by congenital anteromedial subcostosternal defects.3
Two-thirds of all CDHs can be detected on routine fetal ultrasound; the mean gestational age at diagnosis is 22 to 24 weeks,1,2 Infants with CDH often present with respiratory distress after birth, and most of these infants have a Bochdalek hernia. Among the 5% to 25% of children with CDH who have minimal or no symptoms in the neonatal period, CDH may be detected in childhood or even in adulthood. The incidence of CDH in adults is uncertain.4 Once a CDH is identified, surgical repair is indicated to prevent complications, including bowel obstruction or incarceration.
Due to the typically small size of the subcostosternal defect and location, Morgagni hernias are rarely identified on fetal ultrasound.5 Between 34% and 50% of Morgagni hernias are associated with other congenital anomalies such as Down syndrome or atrioventricular septal defect.6,7 Approximately two-thirds of patients with Morgagni hernia are symptomatic in infancy or childhood and present with respiratory symptoms such as recurrent chest infections or chronic cough (56%), gastrointestinal symptoms such as gastric pain and/or nausea and vomiting (19%), or a combination of pulmonary and gastrointestinal symptoms (6.8%) 7-9 Morgagni hernia may be identified in adulthood, presenting with respiratory or gastrointestinal symptoms related to the hernia, or discovered incidentally on an imaging study during workup of an unrelated problem.7-9 Patients with Morgagni hernia may rarely present with intestinal obstruction or respiratory distress requiring emergency surgery.
On chest radiography, Morgagni hernias typically present as a radiolucent retrosternal paracardiac opacity, and, if the bowel is herniated into the chest, a retrosternal air-filled, tubelike opacity may be seen. When a solid organ such as the liver herniates through the diaphragmatic defect, the differential diagnosis includes atelectasis, pericardial fat pad, or intrathoracic tumors such as lipoblastoma and cardiac tumor. If Morgagni hernia is suspected based on chest radiography, an abdominal CT with intravenous contrast should be obtained to confirm the diagnosis.
Once identified, repair of Morgagni hernia should be performed promptly, due to risk of bowel strangulation leading to bowel ischemia or perforation. Minimally invasive surgical techniques, including laparoscopic and thoracoscopic surgery, are the preferred approaches for CDH repair.8. The placement of a patch or mesh may be considered if the defect is large or under significant tension.
Patient Outcome
The patient underwent laparoscopic surgery, which revealed that a portion of the greater omentum had entered the right thoracic cavity through a retrosternal hernial defect. The defect was closed with 3 stitches using a U-shaped suture. At discharge from the hospital 6 days after surgery, the patient’s cough had resolved. Three months later, a noncontrast abdominal CT scan revealed a continuous diaphragm with no evidence of hernia recurrence; minimal fatty tissue, which likely represented the hernia sac; and a small effusion in the right cardiophrenic angle. At most recent follow-up 6 months after surgery, the patient was asymptomatic and had returned to his usual activities.
Endometriosis: addressing the roots of slow progress
(2). Editorial, The Lancet, Volume 404, Issue 10460, p1279, October 05, 2024
A recent paper in Nature Genetics has detailed a comprehensive map of the endometrium across the menstrual cycle, uncovering new information on the processes that might be involved in endometriosis.
Endometriosis is a chronic condition that occurs when tissue similar to the endometrium grows outside of the uterus. Symptoms vary, but many women experience severe period pain along with other gynaecological and systemic symptoms such as heavy bleeding, difficulty conceiving, and fatigue.
Cellular mapping could help determine the cause of endometriosis and might pave the way for novel diagnostics and therapeutics. Although this development is promising, basic science is a long way from reaching the clinic.
Termed the missed disease, owing to its unknown cause and long delays in diagnosis, endometriosis affects 10% of women of reproductive age globally, but standards of care are unacceptably poor. Diagnosis is essential to receiving treatment but receiving it takes too long. In the UK, 58% of women reported making multiple visits to their care provider before any investigations for endometriosis were undertaken, with average waits of 7–9 years to diagnosis globally.
The condition’s effect on quality of life is substantial, with many women experiencing chronic pain (more than two-thirds of women in the USA report missing school or work as a result), along with high levels of comorbid anxiety and depression. Definitive diagnosis is by laparoscopy, which is expensive and difficult to access as it requires surgical intervention. As a result, there are wide disparities in access, with those from minority populations disproportionately affected.
In a multi-country meta-analysis, Black women were 50% less likely to receive a diagnosis than were White women. Data from low-income and middle-income countries are sparse. Although high rates of diagnosis following laparoscopy have been reported (up to 48% in Nigeria), prevalence is often said to be lower than in high-income settings, suggesting gaps in diagnosis are underestimating prevalence.
Management broadly includes surgical removal of tissue or treatment with hormonal therapies or analgesia—options that have remained largely unchanged for decades and target symptoms only, to varying degrees of effectiveness.
There are often long waiting times to surgery, and recurrence rates of up to 50% within 5 years. Hormonal therapies are often halted due to side-effects and cannot be used if a woman desires to conceive.
Consistently poor outcomes for women with endometriosis highlights the profound effect of the women’s health gap. Decades of neglect from funding and research has stifled medical innovation, and gender bias and sexism prevent women from receiving optimal care. Menstrual pain in particular is widely normalised, which—along with taboos on discussing periods and other symptoms such as painful intercourse—means that many women delay seeking medical intervention, or never seek it at all. If they do seek help, symptoms are frequently dismissed or belittled. There are volumes of data to this effect from high-income countries, but data are urgently needed from low-income and middle-income countries too. Advancing basic science is important because it is nearly impossible to develop better treatments or diagnostics without a better understanding of the natural course of endometriosis. But no amount of medical innovation will change outcomes if women are prevented from accessing care.
To improve outcomes for endometriosis, all governments need to formulate plans that not only include measurable targets and interventions across the care pathway but also address the need to change the culture around women, pain, and periods.
Hypothyroidism
(3). Peter N Taylor, et al, The Lancet, Volume 404, Issue 10460p1347-1364October 05, 2024
Summary
Hypothyroidism, the deficiency of thyroid hormone, is a common condition worldwide. It affects almost all body systems and has a wide variety of clinical presentations from being asymptomatic to, in rare cases, life threatening.
The classic symptoms of hypothyroidism include fatigue, lethargy, weight gain, and cold intolerance; however, these symptoms are non-specific and the diagnosis is typically made on biochemical grounds through serum thyroid function tests.
The most common cause of hypothyroidism is chronic autoimmune thyroiditis (Hashimoto’s thyroiditis), although other causes, including drugs (such as amiodarone, lithium, and immune checkpoint inhibitors), radioactive-iodine treatment, and thyroid surgery, are frequent. Historically, severe iodine deficiency was the most common cause.
Reference ranges for thyroid function tests are based on fixed percentiles of the population distribution, but there is increasing awareness of the need for more individualised reference intervals based on key factors such as age, sex, and special circumstances such as pregnancy.
Levothyroxine monotherapy is the standard treatment for hypothyroidism; it is safe and inexpensive, restores thyroid function tests to within the reference range, and improves symptoms in the majority of patients.
However, 10% of patients have persistent symptoms of ill health despite normalisation of thyroid function tests biochemically and a substantial proportion of patients on levothyroxine have thyroid-stimulating hormone concentrations outside the reference range.
Ongoing symptoms despite levothyroxine treatment has led to some patients using liothyronine or desiccated thyroid extract. Taken together, these factors have led to intense debate around the treatment thresholds and treatment strategies for hypothyroidism.
Sepiapterin: A potential new therapy for phenylketonuria
(4). Cary O Harding et al. the Lancet, Volume 404, Issue 10460p1284-1286October 05, 2024
PAH deficiency, although a rare inborn disorder of metabolism, is among the most common disorders detected through newborn screening, with a global prevalence of approximately 1:24 000 livebirths and an estimated half a million individuals affected worldwide.
PAH deficiency is also colloquially known as phenylketonuria because of its association with urinary phenylpyruvate excretion, a detectable phenotype that was used clinically for diagnosis before the advent of amino acid analysis and measurement of blood L-Phe. PAH deficiency impairs the conversion of the essential amino acid L-Phe to L-Tyr and causes accumulation of Phe in the blood and, more critically, in the brain.
Through mechanisms that are incompletely understood, increased Phe concentration in the brain in people with untreated PAH deficiency is associated with acquired microcephaly, growth failure, seizures, and severe global developmental disability.
Dietary Phe restriction initiated during infancy prevents the major manifestations of the disease, but non-adherence to the complicated and unpalatable diet therapy is commonplace during adolescence and adulthood. Chronically increased blood Phe concentration is associated with inattention, impaired memory, disturbed executive function, and psychiatric disorders.
PAH requires the reduced pterin cofactor, tetrahydrobiopterin (BH4), for activity. BH4 is both synthesised de novo and recycled through a salvage pathway to support Phe hydroxylation.
In a subset of individuals with PAH deficiency, dependent on the specific PAH variants in the patient, treatment with oral sapropterin, a synthetic form of BH4, increases endogenous Phe metabolism rate and decreases blood Phe concentration.
This therapy might reduce, but generally does not eliminate, reliance on the low Phe diet in 20–50% of individuals with PAH deficiency. The alternate biopterin precursor, sepiapterin, is converted into BH4 via the biopterin salvage pathway5 and has greater bioavailability than sapropterin.
A previous open-label, phase 2, randomised, controlled, dose-finding trial showed decreased blood Phe concentration after oral sepiapterin administration.
Scolex on the Brain: Intraventricular Neurocysticercosis
(5). Olivia Mobarakai et al, Annals of Internal Medicine: Clinical cases, Volume 3, Number 10, https://doi.org/10.7326/aimcc.2023.1307
Abstract
Neurocysticercosis is a parasitic infection caused by the pork tapeworm Taenia solium. Intraventricular neurocysticercosis develops when cysticerci become lodged in the ventricular outflow tract, which occurs in 10% to 20% of cases. Identification of a scolex within a cystic lesion is pathognomonic. Neurosurgical consultation is recommended to determine the appropriate operative course. When possible, removal of cysticerci using an endoscopic approach is recommended. We present a case of a 22-year-old woman from Honduras who was found to have obstructive hydrocephalus secondary to intraventricular neurocysticercosis and was treated successfully with shunt placement followed by medical therapy.
Case report
A 22-year-old woman from Honduras presented with a 5-day history of throbbing headache with neck stiffness and photophobia. Findings of a lumbar puncture revealed normal glucose level, protein level of 74 mg/dL (reference range, 15–45 mg/dL), and leukocyte count of 420 μL (reference range, 0–5 μL) with 60% eosinophils (reference range, 0–5%). Magnetic resonance imaging (MRI) scan of the brain revealed a cystic lesion of the fourth ventricle with a scolex along the superior aspect and prominence of the lateral and third ventricles with hydrocephalus (Figure 1). This combination of findings—cysticerci in the ventricular cavity, obstructive hydrocephalus (Video 1), and symptoms of increased intracranial pressure—is diagnostic for intraventricular neurocysticercosis (1). When possible, a surgical approach for removal of intraventricular cysts is recommended (2). Surgical options include neuroendoscopic removal, internal drainage, or craniotomy. Extraction of ventricular cysts via neuroendoscopic removal is preferred for obstructing and most nonobstructing cysts on the basis of the rational that retained cysts serve as reservoirs of released antigen that drives the host’s inflammatory response, resulting in ventriculitis, fibrosis, and hydrocephalus (3). Most unremoved cysts still resolve without sequelae after treatment; however, there is an increased risk for recurrence and shunt malfunction with placement of shunts alone (3). In this case, neurosurgery favored a less-invasive approach, and the patient ultimately underwent ventriculoperitoneal shunt placement followed by treatment with dexamethasone and a 14-day course of albendazole. At 3-, 6 -, and 12-month follow-up visits, MRI showed resolution of hydrocephalus. Although most cases are managed with permanent shunting, our patient developed a shunt infection that necessitated shunt removal at 15 months. Annual surveillance MRI continues to demonstrate resolved ventriculomegaly.

Fig (1). Shown is a cystic lesion of the fourth ventricle with a scolex (arrow) along the superior aspect and prominence of the lateral and third ventricles with hydrocephalus.
Patient with Painful and Destructive Nasal Mass
(6). Busra N. Delikkaya etal, JAMA Otolaryngol Head Neck Surg. Published online October 10, 2024. doi:10.1001/jamaoto.2024.3317
Case
A male patient in his mid-30s presented with a 4-week history of progressively increasing pain and swelling involving the bridge of their nose. Physical examination revealed a bulbous subcutaneous mass involving the upper nasal dorsum, with erythema of the overlying skin. They denied fever, chills, sweats, or recent local trauma. Computed tomography scan revealed an irregular rim-enhancing fluid collection in the soft tissues along the anterior margin of the nasal bones measuring approximately 2.1 × 2.1 × 1.4 cm, which appeared to abut the right and left nasal bones with marginal erosion (Figure 1A). Incision and drainage were performed, revealing a white cottage cheese–like discharge. An antibiotic regimen was prescribed, and the patient was discharged.
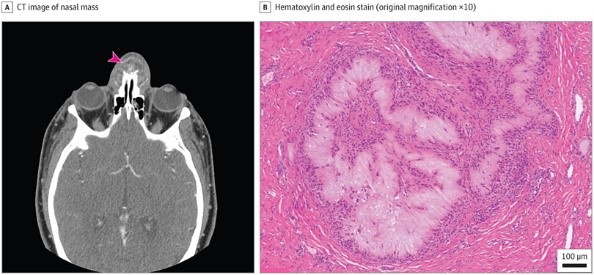
A, Computed tomography (CT) image of the nasal mass displayed an irregular rim-enhancing fluid collection in the soft tissues along the anterior nasal bones (arrowhead). B, Hematoxylin-eosin (original magnification ×10) of the nasal mass showed aggregates of amorphous to fibrillary, pale eosinophilic material, surrounded by palisading histiocytes and multinucleated giant cells.
At follow-up, the patient revealed that the erythema and tenderness had initially improved but then had worsened over the last few days. The patient was taken for surgical excision and debridement of the presumed nasal abscess. During the operation, the surgeons noted that the lesion possessed a capsule and was adherent to the bony/cartilaginous junction with significant scarring and bony remodeling, and a white cottage cheese–like material was expressed upon dissection of the lesion.
Histological sections from the lesion showed ulcerated squamous epithelium with acute and chronic inflammation. The underlying soft tissue and bone contained numerous collections of pale eosinophilic, amorphous to fibrillary material which was surrounded by palisading histiocytes and multinucleated giant cells (Figure 1B).
What Is Your Diagnosis?
- Pseudogout
- Rheumatoid nodule
- Granulomatous polyangiitis
- Tophaceous gout
- Discussion
Diagnosis: Tophaceous gout
Discussion
Gout is the most common form of microcrystalline arthropathy. It is triggered by either an excessive production of uric acid or a decrease in its renal excretion. Sustained elevation in uric acid levels leads to accumulation of mono-sodium urate crystals in various tissues and organs. These crystals incite inflammation when ingested by neutrophils, triggering recurrent bouts of gouty arthritis, and potentially progressing to tophaceous gout over time.
Tophus formation commonly occurs within or near joints, bursae, and tendon sheaths, often eroding bone and leading to distinctive lytic lesions. In the head and neck region, tophi are most commonly found in the helix of the outer ear but can also rarely occur in other areas such as the middle ear, nasal bridge, nasal cavity, and larynx, leading to diverse symptoms from hearing loss to stridor. Gouty involvement of the cricoarytenoid, temporomandibular, and sternoclavicular joints has also been documented.
The diagnosis of gout relies on identifying negatively birefringent urate crystals in joint fluid or gouty tophus samples under a polarized light microscope (Figure 2). Important differential diagnoses include conditions like pseudo-gout, rheumatoid nodule, and immune-mediated systemic diseases like granulomatosis with polyangiitis as they may exhibit similar otolaryngological manifestations, such as nasal bridge collapse, inflammation of the parasinus and sinuses, mucosal ulceration, among others.
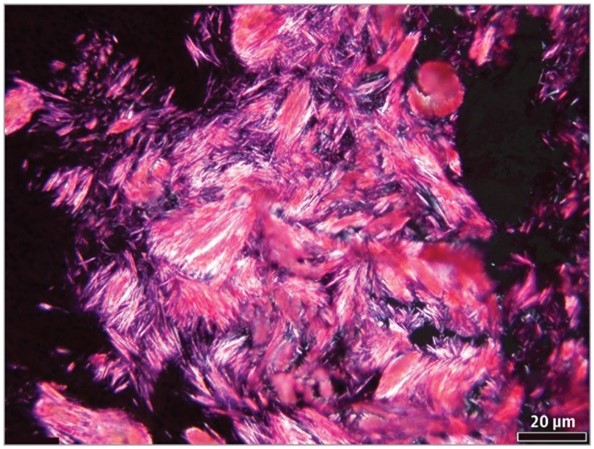
Microscopic examination of the frozen section (unfixed) slides under polarized light reveals needle-shaped crystals consistent with monosodium urate (hematoxylin-eosin; original magnification ×20).
Topheous pseudogout may have remarkably similar histological findings to gout but will show rhomboid-shaped crystals with weakly positive birefringence under polarized light microscopy.6 Rheumatoid nodule manifests as palisading granulomas surrounding areas of fibrinoid necrosis,7 and granulomatosis with polyangiitis will present with necrotizing granulomatous inflammation without birefringent crystals.
When a patient lacks a clinical history of gout or arthritic symptoms, as in the present case, the diagnosis can be particularly challenging. Typically, chronic tophaceous gout emerges following a decade or more of recurrent polyarticular gout; development of tophi without prior episodes of gouty arthritis is uncommon.8 In this case, the patient did not initially disclose a history of gout, but upon further questioning during his postsurgical follow-up visit, he did report having intermittent arthritic symptoms in the past involving his fingers and toes.
Surgical removal of the nasal mass was the preferred approach for this case and similar cases in the literature.2-9 American College of Rheumatology guidelines also recommend that all patients diagnosed with tophaceous gout should undergo urate-lowering therapy together with a 6-month course of anti-inflammatory prophylaxis.
The final diagnosis in this case was of a common inflammatory process that presented in an unexpected location. Tophaceous gout can be recognized or at least suspected on frozen section or routine histologic examination, but confirmatory diagnosis relies on the detection of mono-sodium urate crystals under polarized light microscopy. The clinical history of gout may be helpful but is not always readily available to the pathologist, who must maintain a high index of suspicion when confronted with an inflammatory mass-forming lesion in the head and neck.
Glycogenic Hepatopathy: Images in Clinical Medicine
(7). Åke Sjöholm etal, Published October 19, 2024,N Engl J Med 2024;391:1528,DOI: 10.1056/NEJMicm2403484,VOL. 391 NO. 16,Copyright © 2024
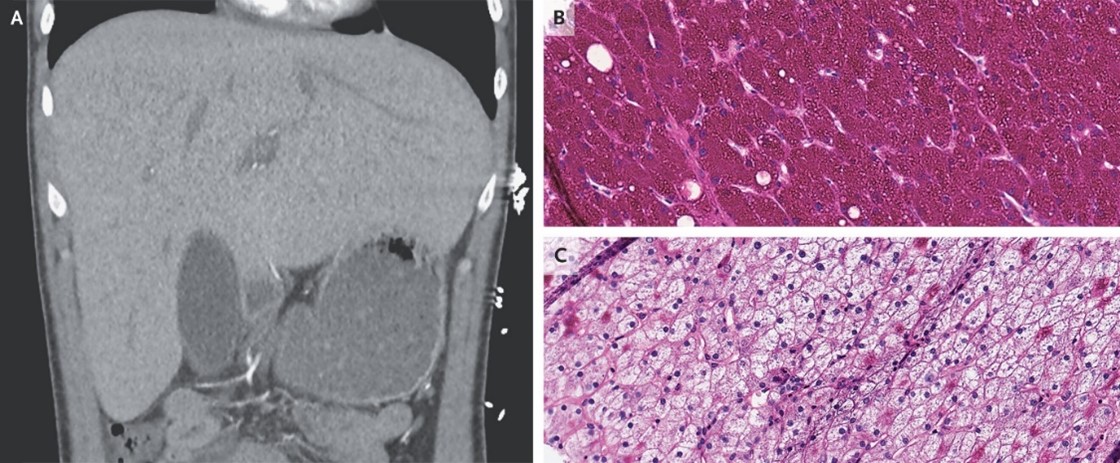
Abstract
An 18-year-old man with type 1 diabetes mellitus who had been admitted with diabetic ketoacidosis had unexpected elevations in aminotransferase levels. CT of the abdomen showed hepatomegaly without parenchymal changes.
Trichuriasis: Images in Clinical Medicine
(8). Guodong Ding etal, Published October 23, 2024,N Engl J Med 2024;391: e34, DOI: 10.1056/NEJMicm2406623,VOL. 391 NO. 16,
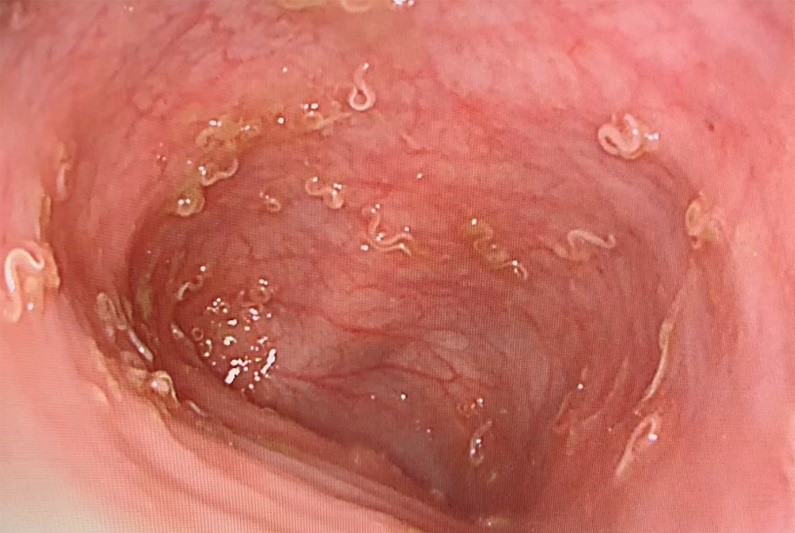
Abstract
A 2-year-old boy presented with a 6-month history of diarrhea and poor weight gain. Tests for infectious diarrhea were negative, but a colonoscopy revealed mobile, white worms on the colon wall
Sustainable practice: Insulin therapy
(9). Tahseen A Chowdhury et al. BMJ 2024; 387 doi: https://doi.org/10.1136/bmj-2024-079425
What you need to know
Insulin prescribing is carbon intensive and leads to considerable plastic waste. Over the past decade, a broader range of medications has been made available to manage type 2 diabetes. If insulin prescribing is required, consider reusable pen devices
More than 500 million people live with diabetes, and around 783 million are expected to have the condition by 2045.1 Of this population, 5% to 15% have type 1 diabetes (T1D).2 Insulin therapy is necessary for people with T1D and for many living with type 2 diabetes (T2D) or other types of the condition (such as gestational diabetes). Waste from diabetes care includes disposables associated with insulin use, testing strips, lancets, needles, and continuous glucose monitoring (CGM) sensors. Many of these items are single use and wrapped in mixed plastics that are often difficult to recycle. Insulin therapy and glucose monitoring generate significant amounts of plastic waste, around 90% of which is packaging.
Only around 9% of general plastic waste is recycled, and many plastics remain in the environment as microplastics, for which there is growing concern about potential harms.4 Insulin prescribing is only a small part of the 133 000 tonnes of plastic waste produced by the UK NHS each year,5 but healthcare professionals can mitigate the effect of insulin therapy on the environment while ensuring clinically appropriate diabetes management.
Why change is needed
England’s diabetes audit data from 2021 show that 21.8% of people with T1D, and 50.1% of people with T2D achieved a glycated haemoglobin of 53 mmol/mol (7.0%) or lower, suggesting that many people live with suboptimal glycaemic control.6 This is despite the fact that, during the same period, more than 7.7 million prescriptions for insulin were written in England, suggesting that despite insulin therapy, glycaemic control is frequently suboptimal.
Should patients treated with direct oral anticoagulants receive intravenous thrombolytics for acute ischaemic stroke?
(10). Faizan Khan, et al, BMJ 2024; 387 doi: https://doi.org/10.1136/bmj-2024-079322 (Published 24 October 2024)
What you need to know
Intravenous thrombolytics are not recommended for acute ischemic stroke in patients who have taken a direct oral anticoagulant (DOAC) within 48 hours before presentation because of the perceived higher risk of intracranial haemorrhage.
The safety of intravenous thrombolytics in this setting is not known because of a paucity of data from adequately powered randomised controlled trials; emerging data of low quality do not show a higher risk of intracranial haemorrhage after thrombolysis in selected patients taking DOACs compared with those not receiving anticoagulant treatment.
Evaluate clinical and imaging characteristics and consider alternative treatments, such as direct endovascular thrombectomy, to determine the risks and benefits for each patient. When available, measuring DOAC plasma levels or reversing DOAC treatment might help individualise decisions about the use of intravenous thrombolytics.
Intravenous thrombolytics, including the recombinant tissue plasminogen activators alteplase or tenecteplase, are the standard treatment for acute ischaemic stroke. They aim to reperfuse the ischaemic brain and reduce disability in eligible patients.
The main criteria for thrombolysis are that the treatment can be administered within 4-5 hours of the onset of stroke symptoms and that there is no intracranial haemorrhage confirmed by imaging before treatment.
About 18% of patients who have an acute ischaemic stroke and might qualify for thrombolysis are also taking direct oral anticoagulants (DOACs) such as factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) or the thrombin inhibitor, dabigatran.
Common indications for treatment with DOACs include stroke prevention in atrial fibrillation, treatment of venous thromboembolism, and coronary and peripheral atherosclerotic disease.
According to guidelines from the American Heart Association/American Stroke Association and the European Stroke Organization, treatment with DOACs within the past 48 hours is a contraindication for thrombolysis because of the perceived higher risk of intracranial haemorrhage
Erysipelas: Images in Clinical Medicine
(11). Nathan I. Wood etal, Published October 30, 2024, N Engl J Med 2024; 391:1632, DOI: 10.1056/NEJMicm2404869,VOL. 391 NO. 17, Copyright © 2024
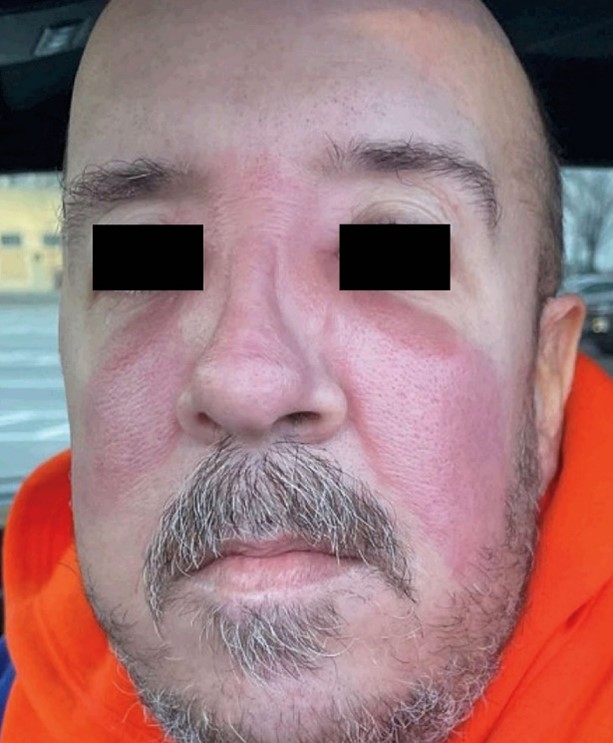
Abstract
A 44-year-old man with infliximab-treated Crohn’s disease presented with a 2-day history of rash on his face. Well-demarcated, warm, erythematous, confluent plaques were seen across the cheeks, nose, and glabella.
Chvostek’s Sign in Familial Hypoparathyroidism: Images in Clinical Medicine
(12). Zhigang Yang, Published October 26, 2024, N Engl J Med 2024; 391: e37, DOI: 10.1056/NEJMicm2406864, VOL. 391 NO. 17, Copyright © 2024
Abstract
A 2-year-old boy was brought to the ED after he had had four generalized seizures in the previous week. Physical examination was notable for brisk tendon reflexes and Chvostek’s sign .

Chvostek’s sign is a clinical sign that indicates neuromuscular hyperexcitability and can be a sign of low blood calcium levels: When the facial nerve is tapped in front of the ear, the facial muscles on the same side of the face will contract abnormally.

