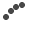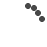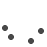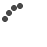Introduction:
After renal transplant due to immunomodulation with drugs and exposure to various viruses, there is increased incidence of post-transplant malignancy. Although kidney transplantation has less Standardized incidence ratio (SIR) of malignancy as compared to lung and liver transplant, they still contribute to overall morbidity. Infact, there are discussion to include post-transplant malignancy as one of the composite outcomes in various clinical trials1. The overall cumulative incidence of any de-novo malignancy in a UK study population shows that transplanted patients have an increased risk2. In most of the western countries after cardiovascular disease, cancer is the most common cause of death with a functioning graft3.
Incidence and mortality of malignancy:
Most of the malignancies occur de novo and less than 1% of the malignancies are pre-existing before transplantation. Cancer transmission from donor is rare although it is important to take informed consent prior to transplantation. Cumulative incidence of malignancy ranges from 10-15% after 15 years of transplant.4The incidence varies with respect to type of cancer. The overall SIR(standardized incidence ratio) for various cancers is high in renal transplant recipients. The data from the UK registry show that there has been an increase in the incidence of Non melanoma skin cancers(16.6%), Kaposi’s sarcoma(17.1%), PTLD(12.5%), oral cancers(65%), Anogenital cancers(10%)2. Certain cancers like breast and prostate cancers are very rare post renal transplantation5. Once the cancer is diagnosed the overall all-cause mortality in these patients have increased
Characteristics of post-transplant malignancy:
Cancer occurs at a younger age compared to the general population in renal transplant patients.6The survival of cancer in various stages is also lower as opposed to general population.6 The average latency period for various malignancies is 12 – 36 months after transplantation. The latency period is longer in paediatric transplantation as the lesions of cancer develop in adulthood with the exception of primary EBV associated PTLD.7Traditional risk factors are increasing age, male gender, smoking and prolonged sun exposure are common to post renal transplant patients. Certain cancers like multiple myeloma and renal cell carcinoma due to ADPKD maybe pre-existing in CKD patients itself.8 Certain geographic incidences exists. Hepatocellular Carcinoma due to hepatitis B is more common in Taiwan renal transplant patients due to endemicity of hepatitis B in Taiwan. Europe, North America and Australia record higher incidence of non-melanoma skin cancers, PTLD and Lip cancers. The Middle East and Asian countries report a higher incidence of urogenital cancers and bladder cancer.9
Mechanism of cancer development:
- Oncogenic viruses: Epstein Barr virus is associated with post transplant lymphoproliferative disease. Herpes Simplex virus cause orogenital cancers. Kaposis sarcoma is associated with Human herpes virus type 8. Human papilloma virus as a causative agent for cervical cancers. Hepatits B and Hepatitis C infection cause chornic liver disease and hepatocellular carcinoma.
- DNA damage from sun exposure; This is an important factor for development of skin cancer. DNA damage does not get repaired adequately due to immunosuppression. Azathioprine causes accumulation of 6 thioguanine in skin after exposure to UVA and sensitizes the skin to development of NMSC.10UVA and UVB sunlight induce DNA damage.
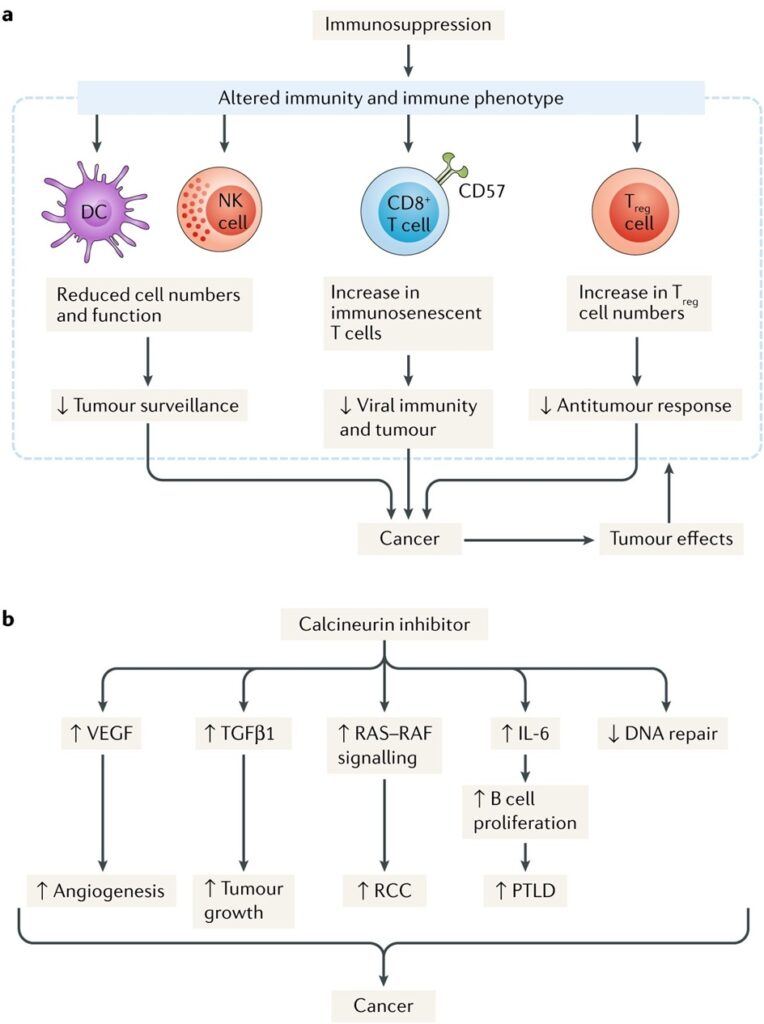
- Immunosuppression: There is not much data to say that one immunosuppressive is more oncogenic than the other. Tacrolimus and cyclosporine increase the production of IL6 and TGF beta which has a role in tumour spread. Cyclosporine has also been shown to inhibit the DNA replication11. Polyclonal agents like ATG and alemtuzumab(anti CD 152) are known for their prolonged T cell depletion and alter the immune homeostasis which in turn predispose to increase incidence of cancer.12 mTOR inhibitors have antiproliferative effects by causing cell cycle arrest and promoting apoptosis.13
The figure below summarizes the pathogenesis of malignancy in renal transplant recipients.
Common cancers after renal transplantation:
Skin cancer – screening and prevention:
It is the most common type of cancer in renal transplant population. Squamous cell carcinoma, Basal cell carcinoma,Kaposi’s sarcoma, malignant melanoma are the common skin cancers encountered. Non melanoma skin cancers account for 90-95% of all the skin cancers.14 Squamous cell carcinoma is more common in SOT recipients than basal cell carcinoma. The risk of Squamous cell carcinoma is 250 times as compared to the general population.14 In general population basal cell carcinoma is more common than squamous cell carcinoma. But a study from Ireland published in 2019, quoted a decline in the overall incidence of keratinocytic carcinoma in renal transplant recipients.15Both SCC and BCC have sun exposure as a risk factor. Other risk factors from the same Irish study include pre transplant skin lesions, thoracic organ transplant, white race, age > 50 years, post-transplant premalignant skin lesions.15UVA and UVB rays have been implicated in pathogenesis of skin cancer. The intensity of the UV rays is peak in the afternoon and the rays have to travel less distance to reach the individual at noon. Hence avoidance of peak noon and 2 hours later is advised for all renal transplant recipients. Sun screen with SPF of 50 and 5 stars rating to prevent exposure to UVB and UVA is advocated for all patients. The DNA damage induced by the UV rays is not able to get repaired due to immunosuppressive medications. UV protective clothing like silk is better than cotton as finer the weave better is the protection. The treatment of SCC and BCC are wide local skin excision. Topical treatment for in situ carcinoma and small tumours include topical fluorouracil and imiquimod, photodynamic therapy and electrodessication. Patients should be warned to screen themselves by skin examination and report if any new lesion occurs. For metastatic SCC, systemic chemotherapy and immunotherapy are recommended. BCC is a local skin tumour and it rarely spreads.
Kaposi’s sarcoma and management:
The incidence of Kaposi’s sarcoma is more in SOT recipients especially in few racial groups like Mediterranean, Africa and Arabian population; with incidence of 100 times more than in general population. It occurs due to reactivation of Human Herpes virus 8. Dissemination with visceral involvement is common and it indicates a bad prognosis. Kaposi’s sarcoma has excellent response to CNI withdrawal and even regression of the malignancy is reported after switch to mTOR inhibitors.16,17The study from NEJM in 2005 showed that 3 months after initiation of mTOR inhibitors all the lesions of kaposi’s disappeared and 6 months later biopsy of skin lesion showed resolution of the cancer.16
Malignant melanoma:
The incidence of malignant melanoma is 5 to 8 times as compared to general population. Studies show poor prognosis of the melanoma in renal transplant patients. Pre transplant melanoma, white race, age>50 years are the main pre transplant risk factors. Surgical wide excision is the treatment of choice.18
Cervical cancer and screening strategies:
Carcinoma cervix accounts for 3% of all post-transplant malignancies in renal transplant recipients and it is the most common cancer after skin cancer in women.19 Oncogenic virus mainly human papilloma virus mainly 16 and 18 are responsible for cervical cancer in many of the patients. Cervical intra epithelial neoplasia is 16 times more common in high risk transplant individuals due to HPV than age matched controls.20 Low risk HPV infection causes viral warts and high risk HPV infection are associated with cervical intra epithelial neoplasia.
Routine cancer screening is beneficial in general population and the same is extrapolated to the transplant patient. WHO recommends HPV DNA testing as first choice of screening for cervical cancer prevention. Males and females aged between 9-25 years are recommended universally for HPV vaccination. Ideally HPV vaccines are recommended prior to renal transplant as the immunological titres of antibody are better prior to transplant. Many centres report HPV vaccination in kidney transplant recipients with variable efficacy.21,22
Carcinoma of the urinary tract:
Renal transplant patients have 4 fold increases in the incidence of bladder and renal carcinoma. The incidence of bladder cancer is also increased in those with BK virus infection.23 The idea that BK virus can cause carcinoma of the urinary tract has been found when BK virus genome has been found to be integrated with cancer genes in few urothelial carcinoma in few studies.24But there are no casual associations between BKV and urothelial cancer. They are limited to case series and reports only. Carcinoma of the prostate is not increased in renal transplant population. There are case reports describing association between BK virus and Prostate cancer.25
Renal carcinoma and Acquired cystic disease:
The presence of ACD is more common in long term dialysis patient more than 3 years. Around 20% of ACD patients will progress to renal cell carcinoma. Renal cancer is around 8 times more common in renal transplant recipient. Routine screening for renal cancer is not recommended for transplant patients as per the American society of transplantation.26
Post-Transplant lymphoproliferative disease: (PTLD)
PTLD is seen to affect 10% of all solid organ transplants. 90% of PTLD are associated with Epstein Barr virus (EBV) infection. EBV is very common infection in childhood and most of the children have been exposed to EBV by adulthood. After renal transplantation, there is reactivation of latent B cells due to T cell immunosuppression. Most PTLD are of the B cell type (95%) and EBV related. The cumulative incidence of PTLD in the first 10 year of transplant is around 1-2% of adults and 3% in paediatric transplants.27 There has been decreasing incidence of PTLD in Australia and New Zealand registry from 1995-2000 to 2005 by 8%.27 PTLD has bimodal distribution after transplant and incidence has been to highest in first 12 months and again later after 5 years only. The late PTLD are most often EBV negative.28The increase in the incidence of PTLD in paediatric recipients is due to primary EBV infection which occurs in the younger population. Overall mortality in PTLD is 50%. The risk factors for PTLD in renal transplant recipients are younger age, male gender, use of T cell depleting agents, negative EBV recipient with EBV positive donor, alemtuzumab use as induction agent are all risk factors for PTLD.27 The use of costimulatory blockade belatacept has been associated with development of PTLD especially cerebral PTLD.29The most common presentation of PTLD with high fever with night sweats and weight loss. The risk of mortality is 14 times higher after PTLD onset in renal transplant patients. Rituximab and use of cyclophosphamide, vincristine, prednisolone, doxorubicin have improved the overall survival at 60%.30
Treatment of post-transplant malignancy:
The general treatment for post treatment malignancy is reduction of immunosuppression and conversion or addition of mTOR inhibitors. mTOR inhibitors have anti-tumour effect by causing cell cycle arrest and hence it helps in reducing the growth of tumours. Specific treatment of individual malignancy depends on the cancer and its type. In astudy published in JASN 2006, sirolimus alone as compared to sirolimus, cyclosporine therapy was associated with decrease median time to BCC, non-skin cancers and SCC too.31In the CONVERT trail, 130 renal allograft recipients 6 to 120 months post-transplant who receiving cyclosporine or tacrolimus, were randomized to get sirolimus or to continue tacrolimus. The study demonstrated significantly lower malignancy rates at 12 and 24 months after transplant with no difference in biopsy proven rates of rejection and patient survival.32The use of immune check point inhibitors in renal transplant patients is not recommended outside case reports due risk of immune activation and rejection.
Waiting time for re-transplantation
Penn in 1993 reported the time between pre transplant cancer occurrence and transplant influences the risk of recurrence after transplant.33 It was one of the seminal papers published in those times. But it added on to mortality of dialysis patients waiting in the list requiring a comprehensive decision of individual cases with medical oncology team.
Low risk breast cancer (stage1) and ductal carcinoma in situ for carcinoma breast no waiting time for transplant patients is needed. Stage 2 cancer breast require 1-2 years waiting time and Stage 3 cancer breast require 3-5 years waiting time before transplant. Stage 4 cancer breast patients are not candidates for renal transplant.34
Low risk colorectal cancer(in-situ cancer and Stage 1 cancer) have waiting time of 1 year. Stage 2 colorectal cancer has waiting period of 2 years. High grades of cancer have waiting time of 5 years and metastatic colorectal cancer are not considered to be transplant candidates.34
Cancer prostate with Gleason score less than 6 have low risk and no waiting time prior to transplant is recommended. High volume or those with intermediate to high risk cancer prostate with Gleason score of more than 7 and PSA>20ng/ml, waiting time for transplant is atleast 6 months after surgery if the disease is stable. There are recommendations to even consider transplant in metastatic cancer prostate after castration if there is cancer regression.34
Renal cell carcinoma T1a and T1b have no waiting time for renal transplantation. T2 RCC has waiting time for 2 years. T3 N0 M0 and T4 N0 M0 cancer have waiting time for minimum 2 years and then reassess before transplant. Any node positive any stage RCC renal transplant is contraindicated.34
Low and intermediate bladder cancer(solitary, <3cm, low grade) risk have waiting time for 6 months. High risk bladder cancer (multifocal, tumour>3cm,high grade T1 tumour) have waiting time of 2 years to assess the cancer activity stages.34
Basal cell carcinoma does not require waiting time for renal transplant as it is a local tumour without metastazing potential. Malignant melanoma in situ and those with breslow depth <1mm require waiting time of 2 years prior to renal transplant. Malignant melanoma with depth more than >1mm and multifocal tumour require prolonged waiting time of 5 years. Sqaunous cell carcinoma with nodal disease a waiting time of 5 years is suggested and so do tumours with peri neural invasion. High risk SCC(>2cm) with poor differentiation need a waiting time of 2 years prior to renal transplantation.34
Adoptive immunotherapy:
It is a modality to consider if conventional treatment does not work for PTLD, lymphoma and leukaemia’s. The use of adoptive immunotherapy in solid organ transplant is controversial. It uses EBV specific cytotoxic T lymphocytes or donor lymphocyte infusion in attempt to prevent EBV proliferation in the B cells. Most of the data come from case series and retrospective reports from hematopoietic transplants. The same case series and reports quote remission in about 52-75% of the patients. The major complication of adoptive immunotherapy is acute and chronic grafts versus host disease.35 Modifications of adoptive immunotherapy to include calcineurin resistance in the gene of T cells have been tried.
Screening of cancer in transplant recipients: It is very essential to prevent cancer in transplant population to improve their overall survival. Routine screening for all cancers in not recommended and for few cancers the recommendations vary from one institute to another.36
Conclusion:
Cancer in a renal transplant patient can devastate their life. The thin balance of immunosuppression reduction and graft rejection has to be looked into a transplant patient when considering “cure” for a cancer. Many guidelines are needed to ascertain the need for screening strategies in renal transplant patients.
References:
- Tong A, Gill J, Budde K, Marson L, Reese PP, Rosenbloom D et al. SONG-Tx Investigators: Toward establishing core outcome domains for trials in kidney transplantation: Report of the standardized outcomes in nephrology-kidney transplantation consensus workshops. Transplantation 101: 1887–1896, 2017.
- Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010 Aug;10(8):1889-96.
- Krynitz B, Edgren G, Lindel€of B, Baecklund E, Brattstr€om C, Wilczek H, Smedby KE: Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008–A Swedish population-based study. Int J Cancer 2013;132: 1429–38.
- Kasiske BL, Snyder JJ, Gilbertson DT, Wang C: Cancer after kidney transplantation in the United States. Am J Transplant 2004;4: 905–13.
- Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR: Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: A cohort study of 15,183 recipients. Am J Transplant 2007; 7: 2140–51.
- Miao Y, Everly JJ, Gross TG, Tevar AD, First MR, Alloway RR, Woodle ES. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009 May 15;87(9):1347-59.
- Van Amstel, Sophie Ploos, and Judith L. VogelzangMD. “Substantial long-term risk of cancer in survivors of pediatric end-stage renal disease.” Effects of initiating chronic renal replacement therapy in children, now and later in life: Data from the LERIC cohort and ERA-EDTA Registry: 83.
- Wong G, Hayen A, Chapman JR,Webster AC,Wang JJ, Mitchell P,Craig JC: Association of CKD and cancer risk in older people.J AmSocNephrol 2009;20: 1341–50.
- Lai MN, Wang SM, Chen PC, Chen YY, Wang JD: Population based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst 2010; 102: 179–86.
- McGurgan IJ, McGuigan C: Nonmelanoma skin cancer risk awareness in azathioprine-treated myasthenia gravis patients. Brain Behav 5: e00396, 2015
- Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, Hofbauer GF, Dotto GP: Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature 2010;465: 368–72.
- Cherikh WS, Kauffman HM, McBride MA, Maghirang J, SwinnenLJ, Hanto DW: Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation 2003; 76: 1289–93.
- Mossmann D, Park S, Hall MN: mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer 2018; 18: 744–57.
- Mittal A, Colegio OR: Skin cancers in organ transplant recipients. Am J Transplant 2017; 17: 2509–30.
- Menzies S, O’Leary E, Callaghan G, Galligan M, Deady S, Gadallah B, Lenane P, Lally A, Houlihan DD, Morris PG, Sexton DJ, McCormick PA, Egan JJ, O’Neill JP, Conlon PJ, Moloney FJ. Declining incidence of keratinocyte carcinoma in organ transplant recipients. Br J Dermatol. 2019 Nov;181(5):983-91.
- Sunil M, Reid E, Lechowicz MJ: Update on HHV-8-associated malignancies. Curr Infect Dis Rep 2010; 12: 147–54.
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005 Mar 31;352(13):1317-23.
- Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, Paddock L, Yanik EL, Lynch CF, Kasiske BL, Snyder J, Engels EA: Melanoma risk and survival among organ transplant recipients. J Invest Dermatol 2015;135: 2657–65.
- Sheil AG. Cancer after transplantation. World J Surg. 1986 Jun;10(3):389-96.
- Veroux M, Corona D, Scalia G, Garozzo V, Gagliano M, Giuffrida G et al. Surveillance of human papilloma virus infection and cervical cancer in kidney transplant recipients: preliminary data. Transplant Proc. 2009 May;41(4):1191-4.
- Nailescu C, Nelson RD, Verghese PS, Twombley KE, Chishti AS, Mills M et al. Human Papillomavirus Vaccination in Male and Female Adolescents Before and After Kidney Transplantation: A Pediatric Nephrology Research Consortium Study. Front Pediatr. 2020 Feb 20;8:46.
- Kumar D, Unger ER, Panicker G, Medvedev P, Wilson L, Humar A: Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant 2013;13: 2411–17.
- Gupta G, Kuppachi S, Kalil RS, Buck CB, Lynch CF, Engels EA. Treatment for presumed BK polyomavirus nephropathy and risk of urinary tract cancers among kidney transplant recipients in the United States. American Journal of Transplantation. 2018 Jan 1;18(1):245-52.
- Sirohi D, Smith SC, Agarwal N, Maughan BL. Unclassified renal cell carcinoma: diagnostic difficulties and treatment modalities. Res Rep Urol. 2018 Nov 15;10:205-217.
- Gorish, B.M.T., Ournasseir, M.E.H. & Shammat, I.M. A correlation study of BK Polyoma Virus infection and prostate Cancer among Sudanese patients – immunofluorescence and molecular based case-control study. Infect Agents Cancer 2019;14: 25.
- Scandling, John D. Acquired Cystic Kidney Disease and Renal Cell Cancer after Transplantation: Time to Rethink Screening?. Clinical Journal of the American Society of Nephrology 2007; 2(4):621-22.
- Francis A, Johnson DW, Teixeira-Pinto A, Craig JC, Wong G: Incidence and predictors of post-transplant lymphoproliferative disease after kidney transplantation during adulthood and childhood: A registry study. Nephrol Dial Transplant 2018;33: 881–889.
- Luskin MR, Heil DS, Tan KS, Choi S, Stadtmauer EA, Schuster SJ, Porter DL, Vonderheide RH, Bagg A, Heitjan DF, Tsai DE, Reshef R: The impact of EBV status on characteristics and outcomes of posttransplantation lymphoproliferative disorder. Am J Transplant 15: 2665–2673, 2015
- Larsen CP, Grinyo J, Medina-Pestana J, Vanrenterghem Y, Vincenti F, Breshahan B et al. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation 2010;90: 1528– 35.
- Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, Olthoff KM, Schuster SJ, Nasta SD, Stadtmauer EA, Tsai DE: Treatment of PTLD with rituximab or chemotherapy. Am J Transplant 2006; 6: 569–76.
- Campistol, Josep M Eris, Josette, Oberbauer, Rainer, Friend. For the Rapamune Maintenance Regimen Study Group. Sirolimus Therapy after Early Cyclosporine Withdrawal Reduces the Risk for Cancer in Adult Renal Transplantation. Journal of the American Society of Nephrology 2006; 17(2):p 581-89.
- Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC et al. Sirolimus CONVERT Trial Study Group. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation. 2009 Jan 27;87(2):233-42.
- Penn I. Effect of immunosuppression on preexisting cancers. Transplant Proc 1993; 25: 1380–382.
- Al-Adra DP, Hammel L, Roberts J, et al. Pretransplant solid organ malignancy and organ transplant candidacy: A consensus expert opinion statement. Am. J. Transplant. 2021;21:460–74.
- Merlo A, Turrini R, Dolcetti R, Martorelli D, Muraro E, Comoli P, Rosato A. The interplay between Epstein-Barr virus and the immune system: a rationale for adoptive cell therapy of EBV-related disorders. Haematologica. 2010;95:1769–77.
- Au, E., Wong, G. & Chapman, J.R. Cancer in kidney transplant recipients. Nat Rev Nephrol 2018;14: 508–20.

Dr Balaji Kirushnan
Consultant Nephrologist,
Kauvery Hospital, Chennai