Abstract
Meliodosis is bacterial infection caused by gram negative bacillus called Burkholderia pseudomallei. It is endemic in tropical and subtropical regions of world. Clinical manifestations can vary which include pneumonia, abscess, joint infection, non-healing ulcer and sometimes severe sepsis requiring ICU admission. This article explains the management of a case of melioidosis in our ICU with our multidisciplinary approach.
Introduction
The infective pathogen Burkholderia pseudomallei was first recognized in 1911 in rhinosphere and ground water in tropical and subtropical regions. This can cause infections in animals and humans(1). Entry of organisms into human occur through broken skin or inhalational route. Clinical manifestations were based upon the route of entry. Infection can range from acute illness to chronic latent form, usually occur in immunocompramised individuals. Case fatality rate ranges from 10-50%(2).
Case presentation
A 35 years old female, who was recently diagnosed as diabetes mellitus presented with history of fever with chills and left knee pain, was initially treated with symptomatic OP based therapy in outside hospital. She was afebrile since then. 10 days later, she presented to outside hospital with history of fever with left knee swelling and pain, where she was admitted and diagnosed as left knee septic arthiritis and started on Inj.Piptaz and linezolid. She was intubated in outside hospital for respiratory distress and referred here for further management. On arrival she was in shock, AKI was present. Cultures taken and started on meropenam and doxycyline. MRI left knee showed inflammatory changes with moderate joint effusion. Orthopaediacian opinion was obtained and she was taken for left knee arthoscopy. Cultures were send intra-op. She required 4 sessions of hemodialysis for AKI. Blood and knee joint cultures grew Burkholderia Pseudomallei. Antibiotics were deescalated to Inj.Ceftazidime. She was gradually weaned and extubated to HFNO. She was then shifted to ward. She was discharged with Tab.cotrimoxazole and doxycycline.
Discussion
The global burden of melioidosis was about 165000 cases per year with about 89000 deaths per year(3). Melioidosis commonly occurs in south east Asia, India and northern Australia. The persons who are at increased risk of acquiring melioidosis include patients with diabetes mellitus, alcohol users, patients with chronic lung disease, chronic kidney disease, chronic liver disease or patients with long term steroids or immunosuppressants or after recent exposure to soil or water(4). Zoonotic transmission to humans is rare.
Clinical diagnosis
The incubation period ranges from 5-21 days. Clinical manifestations depends upon the route of entry and the presence of risk factors. Bacteremia occurs in 50% of affected individuals at admission. Pneumonia is the common presentation(5). The clinical manifestations are explained in following figure.
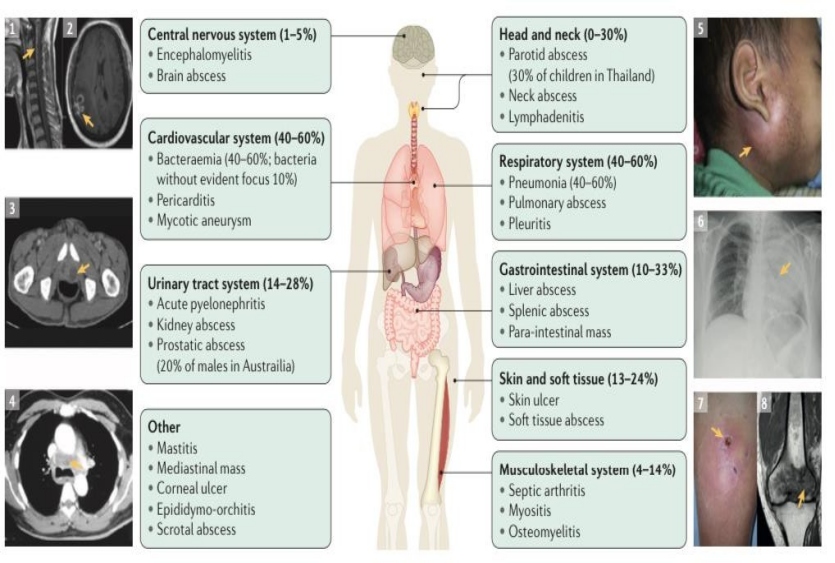 Microbiological diagnosis
Microbiological diagnosis
The mainstay in diagnosis of melioidosis is culture, which can be grown in regular culture media(6). Burkholderia pseudomallei is never a commensal in human, laboratory identification of bacteria warrants infection. Burkholderia pseudomallei is classified as a hazard group 3 pathogen so treating doctors should alert the hospital laboratory if melioidosis is suspected in an admitted patient to ensure that appropriate precautions can be followed. Some laboratories use MALTI-TOF mass spectrometry for bacterial identification as it is more accurate(7). Numerous PCR based tests have been developed for melioidosis identification. The most important amoung them was based on target protein T3SS gene cluster(8).
Management
Early initiation of optimal antimicrobial therapy is key, which along with effective critical care management of sepsis will decrease the mortality to 10%(9). Burkholderia pseudomallei is mostly susceptible to β-lactum antibiotics, doxycycline, chloramphenicol and co-trimoxazole. They are resistant to penicillins, first and second generation cephalosporins, tobramycin, macrolides and polymyxins. Antibiotic therapy is divided into intensive phase and eradication phase.
Intensive phase
Intensive phase includes the use of IV Ceftazidime 2gm IV 6th hourly or IV Meropenam 1gm IV 8th hourly for 10-14 days(10). Dose of meropenam can increased to 2gm IV 8th hourly for neuromelioidosis. Therapeutic response can be slow such that resolution of fever can take upto 9 days. Duration of intensive phase can be prolonged to 4-6 weeks in case of deep seated abscess or osteomyelitis.
Eradication phase
Eradication phase starts from the end of intensive phase for a duration of 3 month. It involves the use of co-trimoxazole with careful monitoring of adverse effects of co-trimoxazole. Occurance of adverse effects needs dose modification or switch to second line drugs which include oral doxycline or amoxy-clav(11). Longer eradication phase of 6 months may be needed in neuromelioidosis and osteomyelitis.
Surgical management
Surgical drainage of abscess is usually needed in case of single large abscess of muscle, liver or prostate(12). Repeated drainage and washouts may be needed in case of septic arthiritis. Mycotic aneurysms will require urgent surgery with replacement of prosthetic grafts. In such case, life long eradication therapy will be needed.
Conclusion
Melioidosis is serious infection caused by gram negative bacilli, more common in patients with diabetes mellitus or on any immunosupperasants. It has wide range of organ involvement. No vaccine is currently available against melioidosis. Adherence to therapy is the key.
Referances
- Kaestli, et al. Out of the ground: aerial and exotic habitats of the melioidosis bacterium Burkholderia pseudomallei in grasses in Australia. Environ. Microbiol. 14, 2058–2070 (2012).
- Cheng, C. & Currie, B. J. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18, 383–416 (2005).
- Limmathurotsakul, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 1, 15008 (2016).
- Currie, J. et al. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop. Med. Int. Health 9, 1167– 1174 (2004).
- Stevens, P. et al. A Burkholderia pseudomallei type III secreted protein, BopE, facilitates bacterial invasion of epithelial cells and exhibits guanine nucleotide exchange factor activity. J. Bacteriol. 185, 4992–4996 (2003).
- Kespichayawattana, Rattanachetkul, S., Wanun, T., Utaisincharoen, P. & Sirisinha, S. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect. Immun. 68, 5377–5384 (2000)
- Stevens, P. et al. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol. Microbiol. 56, 40–53 (2005).
- Egan, M. & Gordon, D. L. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect. Immun. 64, 4952–4959 (1996).
- Sarovich, S. et al. Variable virulence factors in Burkholderia pseudomallei (melioidosis) associated with human disease. PLoS ONE 9, e91682 (2014).
- Hoffmaster, R. et al. Melioidosis diagnostic workshop, 2013. Emerg. Infect. Dis. http://dx.doi.org/ 10.3201/eid2102.141045 (2015).
- Suttisunhakul, et al. Matrix-assisted laser desorption/ionization time-of- flight mass spectrometry for the identification of Burkholderia pseudomallei from Asia and Australia and differentiation between Burkholderia species. PLoS ONE 12, e0175294 (2017).
- Kaestli, et al. Comparison of TaqMan PCR assays for detection of the melioidosis agent Burkholderia pseudomallei in clinical specimens. J. Clin. Microbiol. 50, 2059–2062 (2012).
- Currie, J. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Respir. Crit. Care Med. 36, 111–125 (2015).
- White, J. et al. Halving of mortality of severe melioidosis by ceftazidime. Lancet 2, 697–701 (1989)
- Ooi, F. et al. The condition-dependent transcriptional landscape of Burkholderia pseudomallei. PLoS Genet. 9, e1003795 (2013)
- Morse, P. et al. Osteomyelitis and septic arthritis from infection with Burkholderia pseudomallei: a 20-year prospective melioidosis study from northern Australia. J. Orthop. 10, 86–91 (2013).
 Dr. Dhinesh Raj
Dr. Dhinesh Raj
Critical Care Medicine
Kauvery Hospital, Chennai