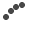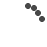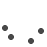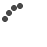Case Report:
76-year-old Mr M (identity concealed) presented to us with Class II-III dyspnea on exertion and three episodes of syncope. His echocardiogram elsewhere showed severe left ventricular outflow tract obstruction. Evaluation by our team revealed a significant hypertrophic obstructive cardiomyopathy (HOCM) with a LVOT gradient of 90 mm-Hg and a severe mitral regurgitation (MR). The basal inter-ventricular septum was 26 mm in thickness. Electrocardiogram showed significant left ventricular hypertrophy with left axis deviation. Chest x-ray showed an LV type of apex with LVH.
He had been advised HOCM correction with mitral valve replacement else-where. We reassured the patient that correction of HOCM was enough and mitral valve may not need an intervention.
After a routine workup, we took him up for a corrective surgery. The options would have been an extended septal myectomy which usually gives a good result in experienced hands versus an alcohol septal ablation which is not done in most centres.
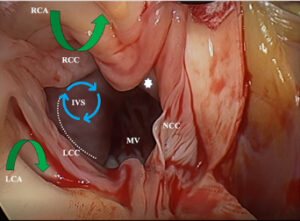
At the start of the surgery, our experienced cardiac anaesthetist performed a detailed transesophageal echo (TEE) that demonstrated the septal thickness from the basal septum, mid septum to the apical septum. The degree of basal septal hypertrophy, C-sept measurement, degree of mitral regurgitation and abnormal papillary muscles was looked into. This gave us a fair idea of the level and amount of septal tissues to be resected.
Once the patient was connected to the heart lung machine, the heart was arrested in diastole, the septum was approached through the aorta after careful retraction of the right coronary leaflet. An extended septal myectomy was performed with care to avoid injury to the conduction bundle of the heart and the chordal apparatus of the mitral valve.

After an adequate resection, the patient was weaned off cardio-pulmonary bypass uneventfully with minimal inotropes and was extubated in 4 hours after surgery. The ICU stay was about 48 hours. A post-operative TEE was used to confirm the adequacy of septal resection and regression of MR. In this particular patient of ours, the basal septum was reduced to 18 mm thickness and the residual MR was trivial in spite of no interventions to the mitral valve.
Extended Septal Myectomy:
 The original surgery advised by Andrew Morrow was to excise a limited bit of septal tissue, but currently we practice an extended septal myectomy, so that a liberal bit of tissue is excised and the left ventricular outflow is completely opened up without any obstruction.
The original surgery advised by Andrew Morrow was to excise a limited bit of septal tissue, but currently we practice an extended septal myectomy, so that a liberal bit of tissue is excised and the left ventricular outflow is completely opened up without any obstruction.
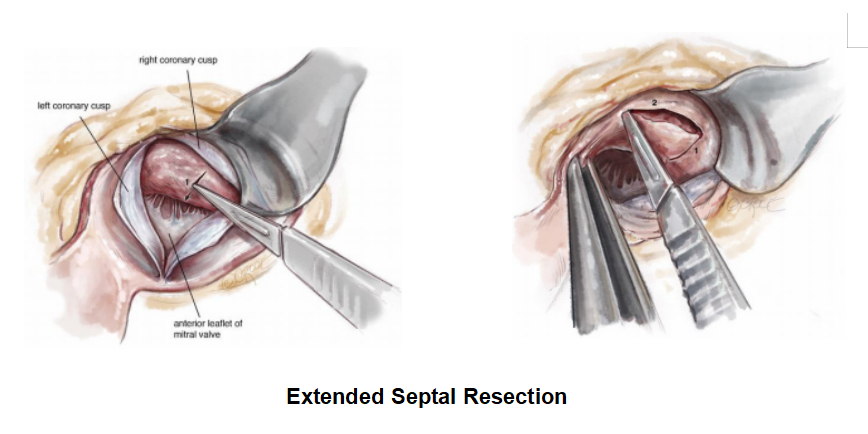
The mitral valve is usually preserved and an adequate resection settles the mitral regurgitation (MR) even if the MR is severe pre-operatively. The pathophysiology of MR associated with HOCM is explained in detail later in this article.
It’s an irony that Dr Andrew Morrow who described this septal myectomy surgery and had performed plenty of them at National Institute of Health, Maryland, was diagnosed with HOCM by Dr Braunwald, his colleague of long time.
Personal Case Series:
The author has performed about 42 of such extended septal myectomy.
Mean Age of Patients : 46 years
Mitral Valve Interventions : 0
Pacemaker Implantation : 1
Mortality : 2 (One patient due to LV dysfunction & one patient due to sepsis.)
HOCM
Hypertrophic cardiomyopathy (HCM) affects 1 in 500 individuals and is grossly under-reported. Schmincke, a German pathologist, first reported the condition in 1907. The modern description of HCM was done by Teare in 1958. Braunwald examined 64 patients and described it as “idiopathic hypertrophic subaortic stenosis” (IHSS).
HCM is an autosomal dominant disorder with 14 genes and 1400 mutations described so far. These genes encode for sarcomere or sarcomere related protein and lead on to exuberant left ventricular hypertrophy. The most commonly involved genes are MYH7, MYBPC3, TNNT2 and TNNI3. Mutation of MYH7 and MYBPC3 genes account for almost 80% of hypertrophic cardiomyopathy. Mutation in various genes coding for the sarcomere proteins leads to ventricular hypertrophy, myocardial disarray and fibrosis. The disease rarely develops before adolescence. Mutation of more than 1 gene (< 5% incidence) may have a severe form of disease. Mutation responsible for HCM is transmitted by autosomal dominance and each offspring of an affected male has 50% chance of inheriting the mutation. Select mutation can demonstrate substantial variability in age-related penetrance, resulting in delayed expression of the phenotype to third decade of life or even beyond mid-life.
HCM is defined as non-dilated LVH in the absence of other cardiac or systemic causes of LVH. The LV wall thickness of > 15 mm by echocardiography or MRI indicates HCM. Subclinical HCM is defined as genotype positive in the absence of phenotype expression. Differential diagnosis includes aortic stenosis, systemic hypertension, athlete’s heart, storage disorders like Pompey’s disease and Fabry’s disease, and Noonan’s syndrome.
HCM is a heterogeneous disorder with diverse manifestations and clinical course. Majority may have normal life expectancy, a few may have life-threatening complications which include ventricular tachy-arrhythmias, sudden cardiac death (SCD), heart failure symptoms and atrial fibrillation with thromboembolism.
Three different clinical types are recognised. (1) Non-Obstructive – These patients have LVOT peak gradient < 30 mm Hg under basal and provocative conditions. (2) Basal Obstructive – These patients have a resting LVOT gradient of > 30 mm Hg. (3) Labile Obstructive – These patients have a resting gradient of < 30 mm Hg but a provocative gradient of > 30 mm Hg. The gradient is usually measured by a transthoracic echo with continuous wave Doppler, or rarely by cardiac catheterisation. Gradient above 50 mm Hg is significant and needs intervention.
Patients with LVOT gradient above 50 mm-Hg at rest or provocation and persistent symptoms of dyspnea and chest pain are referred for invasive management. Amyl nitrite, Valsalva maneuver and isoprenaline are commonly used for provocation.
The clinical course of HCM is usually divided into four stages.
Stage 1: (Subclinical HCM) Patient is genotype positive but yet to develop phenotype expression.
Stage 2: (Classic HCM) The ejection fraction is supra-normal > 65% and late gadolinium enhancement (LGE) shows myocardial fibrosis < 5% of LV mass. 70% of these patients have LVOT obstruction at rest or on provocation.
Stage 3: (Adverse Remodelling) EF 50-65%, LGE 10-15%.
Stage 4: (Overt Dysfunction or End Stage) EF < 50%, LGE > 25%, dilated or restrictive cardiomyopathy, LVOT obstruction may be absent.
The two main issues of clinical relevance in HCM are (1) The pathogenesis, clinical consequences and management of LVOT obstruction and consequent mitral valve involvement. (2) The arrhythmia risk stratification and prevention of sudden cardiac death (SCD).
Left Ventricular Hypertrophy (LVH): LVH is classically described as asymmetric and commonly the inter-ventricular septum is involved. Hence the term asymmetric septal hypertrophy. Basal septum is very commonly involved and involvement of mid-septum and apical septum is quite rare. Hypertrophy can involve the papillary muscles and rarely the right ventricle. Massive hypertrophy is considered when the LV wall thickness is > 3 cm and has important prognostic considerations.
Mitral Regurgitation: Varying degree of mitral regurgitation (MR) is quite common in most patients with HOCM with basal gradients. The various anomalies include:
- Anterior displacement of mitral valve compared to controls.
- Elongated Anterior Mitral Leaflet: (AML) The length of AML exceeds 30 mm compared to 25 mm in controls.
- Excessive area of AML.
- Anomalies of papillary muscles which include hypertrophy, increased papillary muscle heads, anterior displacement of papillary muscles and direct insertion of papillary muscle to AML.
- Degenerative myxomatous changes.
LVOT Obstruction: High velocity jet in LVOT and pressure drop above the aortic valve are considered to pull the AML into the LVOT by Venturi effect aggravating the obstruction. This leads on to systolic anterior motion of mitral leaflet (SAM). The three requisites for LVOT obstruction are:
- Mechanical obstruction of LVOT by asymmetric hypertrophy
- SAM
- Mitro-septal contact
Mid-Ventricular Obstruction (MVO): Mid-ventricular obliteration is caused by marked septal hypertrophy resulting in contact with hyper contractile LV free wall and the papillary muscles in systole. In contrast to sub-aortic obstruction, MR is not a feature of MVO. It occurs in only 5-10% of patients and usually due to heart failure or associated LV apical aneurysm.
Treatment Options:
Conservative and Medical Management
Alcohol Septal Ablation
Septal Resection
Automatic Implantable Cardioverter Defibrillation
The goals of treatment in HOCM are to relieve the obstruction and prevent sudden cardiac death.
The indications for an intervention are when the LVOTO gradient is > 50 mm-Hg, moderate or severe symptoms and recurrent exertional syncope in spite of maximal drug therapy.
Medical Management:
Beta-blockers
Calcium channel blockers
Anti-arrhythmics – amiodarone or disopyramide
Anti-coagulants – if the patient is in atrial fibrillation
Alcohol Septal Ablation:
Alcohol septal ablation was first introduced in 1994 by Sigwart. According to the current AHA guidelines, alcohol septal ablation (ASA) is reserved for patients with significant co-morbidities which preclude a surgery, and in patients who are unwilling for surgery.
Alcohol septal ablation needs expertise and the reduction of septal thickness and regression of MR is unsatisfactory. Due to the scarring of the septum, the incidence of arrhythmias needing an AICD implantation is a bit high.
Extended Septal Myectomy:
Surgery is considered as the gold standard in the treatment of HOCM. An adequate resection receives the LV outflow obstruction completely and relieves the MR. Usually, no intervention is needed for the mitral valve unless there is a gross morphological abnormality in the mitral valve or there is an abnormal papillary muscle attaching directly to the anterior mitral leaflet (AML).
AICD Implantation and Pacemaker Implantation:
HOCM and HCM patients are prone to ventricular arrhythmias and sudden cardiac death. Patients with documented ventricular arrhythmias will need an AICD implantation to prevent sudden cardiac death. Rarely, a dual chamber is implanted to reduce the thickness of the septum and only 15% of patients respond to this therapy.
For further details please contact:
 Dr Anbarasu Mohanraj, MS, DNB, M.Ch, FRCS.
Dr Anbarasu Mohanraj, MS, DNB, M.Ch, FRCS.
Director & Clinical Lead,
Dept of Cardio-Thoracic Surgery,
Kauvery Heart Institute,
Kauvery Hospital, Vadapalani. Chennai.
anbarasu.mohanraj@gmail.com