Hypertensive intracerebral hemorrhage (ICH) is a life-threatening condition characterized by bleeding within the brain due to elevated blood pressure. Various factors, including gender, comorbidities, hematoma side, volume, and location, may influence the prognosis and treatment outcomes of patients with hypertensive ICH.
This study aims to compare the in-hospital management outcomes of hypertensive ICH patients based on these factors, with a specific focus on the use of tranexamic acid (TXA). By examining these parameters in a prospective manner, we aim to gain a deeper understanding of the potential gender-related differences in hypertensive ICH presentation, progression, and prognosticating the outcome.
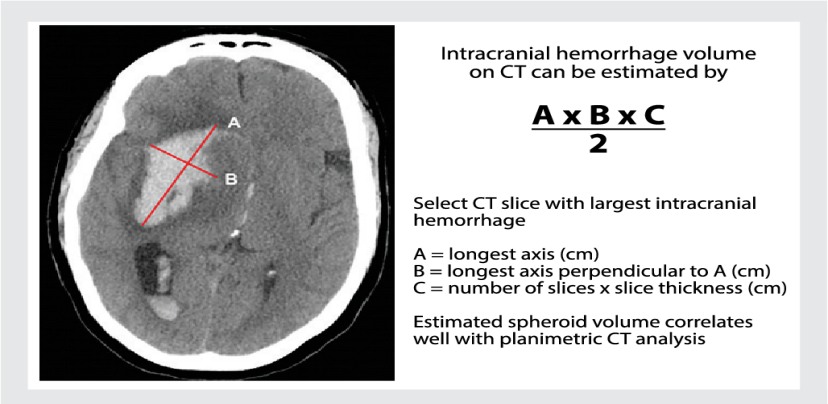
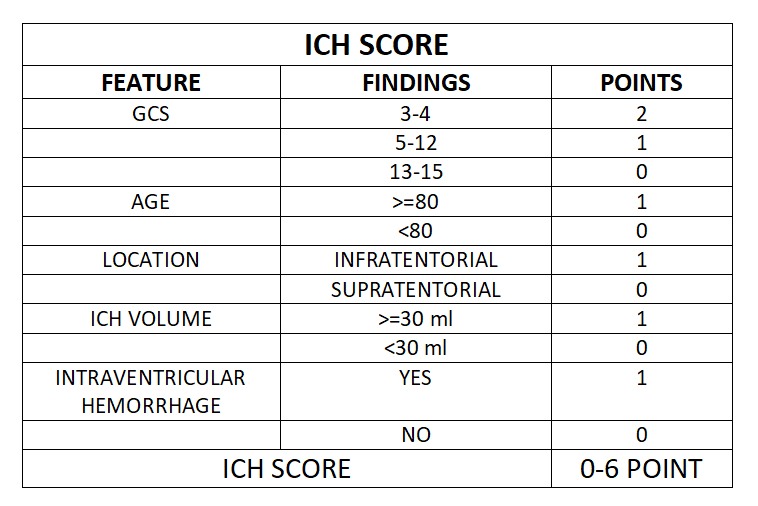
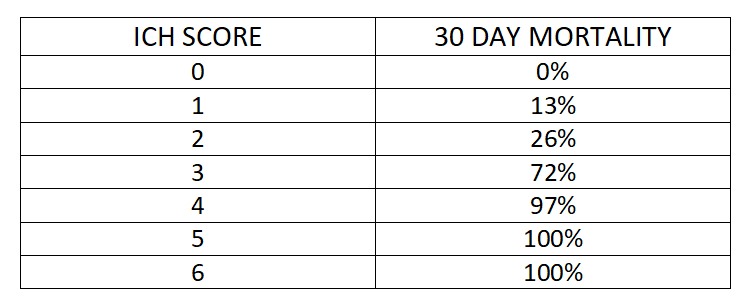
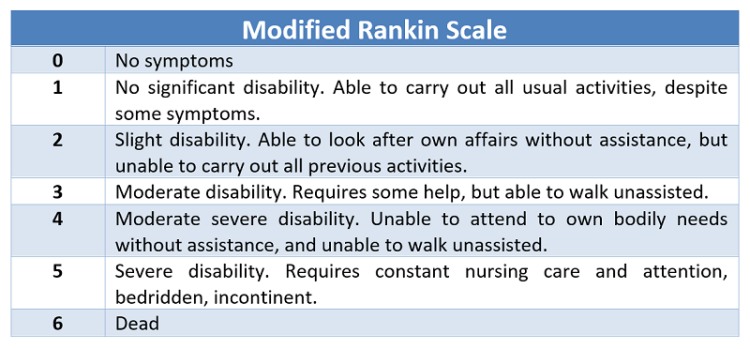
This is a prospective observational study. A total of 100 patients with hypertensive intracranial bleed more than 18 years of age presented within 8 hours are studied. Exclusion criteria includes Age <18, Vascular malformation, Aneurysms, Trauma, Presentation >8hrs, Serum Creatinine >2, Sub arachnoid hemorrhage, Hypersensitivity to TXA . Patients sample are collected prospectively from our hospital and classified into male and female categories. Comorbid conditions documented and imaging will be done where the side and location (thalamic/ gangliocapsular) with volume of hematoma with or without midline shift, presenting GCS and measurement of pupil noted. Using ICH scoring system, patient is scored. Initial management of condition like endotracheal intubation, blood pressure control (SBP<150mmHg, target MAP 65 mmHg) and glycemic control, anti-edema measures taken. For patient with ICH score above 2 tranexemic acid 1 gram IV bolus followed by 1-gram IV Q8H is given. Comparing initial CT with follow up imaging and the management (conservative/surgical) with outcome at 5th day and 30th day assessment done. The mortality rate is compared with the percentage listed by Hemphil.et.al. Sensitivity, specificity, and positive and negative predictive values will be calculated. Disability and functional status will be measured using the Modified Rankin scale.
Primary Outcome:
Did intervention like use of tranexemic acid changed the prognosis of the patient whose 30-day mortality rate percent is high?
Secondary Outcome:
What is the impact of gender in the outcome?
REVIEW OF LITERATURE
- Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al.. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2032–60. 10.1161/STR.0000000000000069
- Meretoja A, Yassi N, Wu TY, Churilov L, Sibolt G, Jeng JS, et al. Tranexamic acid in patients with intracerebral haemorrhage (STOP-AUST): a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2020;19(12):980–7. pmid:33128912
- Li Q, Liu QJ, Yang WS, Wang XC, Zhao LB, Xiong X, et al.. Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke. (2017) 48:3019–25. 10.1161/STROKEAHA.117.017985
- Hemphill JC 3rd, Farrant M, Neill TA Jr. Prospective validation of the ICH score for 12-month functional outcome. Neurology. (2009) 73:1088–94. 10.1212/WNL.0b013e3181b8b332
- Jiang C, Wang J, Wang J, Zhang J, investigators T-I. Rationale and Design of a Randomized, Double-Blind Trial Evaluating the Efficacy of Tranexamic Acid on Hematoma Expansion and Peri-hematomal Edema in Patients with Spontaneous Intracerebral Hemorrhage within 4.5 h after Symptom Onset: The THE-ICH Trial Protocol. J Stroke Cerebrovasc Dis. 2020;29(10):105136.
- Tanaka K, Toyoda K. Clinical Strategies Against Early Hematoma Expansion Following Intracerebral Hemorrhage. Front Neurosci. 2021;15:677744. pmid:34526875
- Yassi N, Zhao H, Churilov L, Campbell BCV, Wu T, Ma H, et al. Tranexamic acid for intracerebral haemorrhage within 2 hours of onset: protocol of a phase II randomised placebo-controlled double-blind multicentre trial. Stroke and vascular neurology. 2022;7(2):158–65. pmid:34848566
- Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391(10135):2107–15. pmid:29778325
- Arumugam A, NA AR, Theophilus SC, Shariffudin A, Abdullah JM. Tranexamic Acid as Antifibrinolytic Agent in Non Traumatic Intracerebral Hemorrhages. Malays J Med Sci. 2015;22(Spec Issue):62–71. pmid:27006639
- Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97(4):771–8. pmid:12405362



 Dr. Silvera Samson Raj
Dr. Silvera Samson Raj Dr. Arunkumar Karthikayan
Dr. Arunkumar Karthikayan