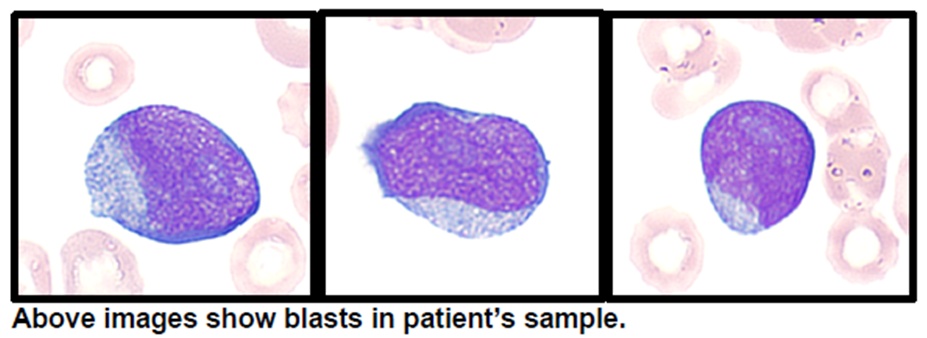
24 years old male came with complaints of shortness of breath, loss of weight (5 kg) and appetite, recurrent fever for the past 1 month. Patient had history of giddiness and palpitations for 1 month there was history of generalised fatiguability for 2 months. Family history revealed aplastic anemia/ AML in elder sibling who died at the age of 14 years. There was history of leukemia in cousin who died at the age of 6 years. On examination, patient had tachycardia, pallor and hepatosplenomegaly. Basic blood investigations revealed – Hb:3 g/dl, WBC: 1300/cu.mm (N-40, L-50, M-10, B-0), platelet count: 92000/ cu.mm. Serum LDH level was 405 U/L. RBC indices, renal function tests, live function tests, coagulation profile, vitamin B12 levels, and urine routine were normal. ANA, direct coombs test, CMV IgM, Parvovirus B19 IgM, Hepatitis B and HIV serology were negative. Peripheral smear revealed pancytopenia with more than 20% blasts. CECT chest revealed smooth interlobular septal thickening in bilateral lower lobes likely due to pulmonary congestion and no evidence of lymphadenopathy. CECT abdomen showed hepatosplenomegaly and no lymphadenopathy. Hematologist opinion was obtained and bone marrow aspiration/biopsy was done. Bone marrow aspirate showed 22% blasts, intermediate to large in size, high N:C ratio, fine chromatin, 0-2 nucleoli, scanty to moderate cytoplasm- Features suggestive of acute leukemia. Flow cytometry was suggestive of acute myeloid leukemia. Bone marrow biopsy showed acute myeloid leukemia with mild focal myelofibrosis. Oncohaem Next generation sequencing (NGS) panel showed pathogenic mutations in SH2B3, BCOR, PHF6 and WT1. Four units of packed red blood cell (PRBC) transfusion was done. He was suggested induction therapy (7+3 therapy with cytarabine and anthracycline). Due to personal reasons, patient wants to proceed with further treatment in outside institute, hence was discharged.
Discussion
AML is a aggressive malignancy of white blood cells with very high mortality rate. Historically the five year overall survival rates (21%) had been dismally low which are comparable to survival rates of aggressive solid tumors of lung, liver and brain (19%). But the prognosis depends on various factors like cytogenetics, type of genetic mutations, type of AML (primary vs secondary AML). Even though the incidence of AML (1.1% of cancer diagnosis) is rare, it is the second most common leukemia after Acute lymphoblastic leukemia (ALL). AML primarily affects middle aged and older adults with the exception of acute promyelocytic leukemia (APL) which occurs in pediatric age group.
Clinical manifestations of AML include mainly symptoms of bone marrow failure and organ infiltration. Bone marrow failure can present as pallor (anemia), infections (WBC involvement) and bleeding tendencies (thrombocytopenia and DIC). Occasionally it may be incidentally detected on a routine blood cell count and smear analysis done for some other indication. Organ infiltration can be in the form of involvement of gums, skin and lymphnodes. Rarely AML can present as solid tumor in the form of myeloid sarcoma. Patients with AML are at increased risk of tumor lysis syndrome which manifest with hyperkalemia, hyperphosphatemia and hyperuricemia. Therefore AML patients should receive good hydration, prophylactic allopurinol and sometimes rasburicase in active tumor lysis syndrome. AML patients can also have symptoms related to leucostasis due to hyperleucocytosis such as stroke like events, vision disturbances, confusion, dizziness etc. Diagnosis typically requires demonstration of >20% blasts in bone marrow aspirate sample by morphology and demonstration of immature blast population and cell surface/ cytoplasmic markers of myeloid lineage by immunohistochemistry/ flow cytometry. AML can also be diagnose with <20% blasts if there are recurrent genetic abnormalities like t(8:21), inv (16).
European Leukemia Network (ELN) prognostic classification system is a useful system for prognostification of treatment of AML. It divides AML into three categories: favourable, intermediate and adverse risk. Patients with NPM1, CEPBA mutations are grouped under favourable risk category. Patients with RUNX1, TP53, BCOR, EZH2, complex karyotypes are grouped under adverse risk category. Mutations of NPM1 if associated with FLT3-ITD is grouped under intermediate category. AML diagnosis is a medical emergency as delay in treatment can lead to increased complications and mortality. Classically AML treatment is with 3 days of daunorubicin along with 7 days of cytarabine (7+3 regimen). With advent in cytogenetics, immunoflowcytometry and genetic mutation testing, targeted therapy based on the mutation type in a particular patient can be planned. For eg, patient with favourable and intermediate prognosis is given gemtuzumab-ozogamicin (monoclonal antibody drug targeting CD33) in addition ti 7+3 regimen. Patients with FLT3 mutations are given midostaurin in addition to 7+3 regimen. CPX-351 has been used in secondary AML. Previously focus was mainly given to the intensive chemotherapy regimen. Now the focus has also been given to consolidation phase treatment also like in ALL to improve the overall cure rates and to decrease relapses. Presence of measurable residual disease (MRD) is associated with poor outcome due to higher chances of relapse even after morphologic remission. Hematopoietic stem cell transplant (HSCT) is the best treatment to reduce relapse post remission. But the benefits of the HSCT in reducing relapse is offset by non relapse related mortality hence it is not considered upfront in AML patients with favourable risk category but it is considered in intermediate/adverse risk category patients in first complete remission. Various targeted therapy based on genotypehas been tested in trials like hypomethylating agents (azacitidine and decitabine), isocitrate dehydrogenase (IDH) inhibitors like ivosidenib and enasidenib, FLT3 inhibitor (gilteritinib), BCL-2 inhibitor like venetoclax. Treatement of AML has evolved drastically in the last decade from standard 7+3 regimen with daunorubicin and cytarabine to targeted therapy based on risk category and molecular (genetic mutation) analysis.
 Dr. Sowmiya
Dr. Sowmiya
DNB Final Year Resident
Department of Internal Medicine
Kauvery hospital Chennai