Case Report
A 40 year old gentleman born out of a non consanguineous marriage via spontaneous vaginal delivery at term was diagnosed to have ventricular septal defect(VSD) at birth. He had history of recurrent fever episodes associated with cough and difficulty in feeding since 4 months of age. At 6 months of age he developed facial puffiness. He was managed as OP with diuretic and Lanoxin, after which he lost follow up. He remained asymptomatic till 17 years of age, when he developed worsening shortness of breath and peripheral cyanosis. On evaluation he was found to have PAH with reversal of shunt – Eisenmengers syndrome due to which he was not advised surgery. Laboratory investigation revealed polycythemia with hemoglobin of 24mg/dl. He underwent periodic phlebotomy for the same. At 22 years of age, he had acute CVA, underwent craniectomy with clot evacuation. Coronary angiogram showed normal coronaries.
In 2021, he was referred here with complaints of gradually reduced urine output with pedal edema and shortness of breath. His blood pressure was 130/80 mmHg, pulse rate 82/min, oxygen saturation 84- 86%. On examination he had grade 3 pan digital clubbing, a pansyatolic murmur at lower left sternal border and diastolic murmur in the pulmonary area. Laboratory findings revealed 5g/day proteinuria, hemoglobin 19g/dl, hematocrit 55.8%, serum creatinine 0.8 mg/dl, uric acid 9.0 mg/dl, total protein 4.9g/dl, albumin 2.6g/dl. He was started on oral steroid along with Losartan 25mg BD,antiplatelet. There was no improvement in his symptoms or protienuria after 1 month of steroid. He was started on Tacrolimus( Pangraf) at an initial dose of 0.5mg BD. On follow up, his urine PCR was found to be the same. Tacrolimus dose was stepped up. He is currently on 1.5mg BD and on regular op follow up. His urine PCR is varying between 5 and 7.
Discussion
Focal segmental glomerulosclerosis (FSGS), a histologic pattern of glomerular injury, defines a number of clinicopathologic syndromes that may be primary (idiopathic) or secondary to diverse causes.[1,2]
Subtypes include classic, perihilar variant,cellular variant, manifesting endocapillary hypercellularity; collapsing variant, in which at least one glomerulus has global collapse and overlying visceral cell hypertrophy and hyperplasia; and tip variant, with segmental lesions involving the tubular pole.[1-4]
Perihilar variant is defined by the presence of at least one glomerulus with perihilar hyalinosis with or without sclerotic lesions at the glomerular vascular pole (perihilar) in more than 50% of glomeruli with segmental lesions. This form has been described in primary FSGS and more frequent in secondary forms from nephron loss or glomerular hypertension (due to obesity, reflux nephropathy, hypertension, cyanosis heart disease, sickle cell disease)usually accompanied by glomerular hypertrophy and mild foot process effacement.
Around 30 and 50% of cyanotic heart diseases can be the cause of a secondary glomerulopathy.[5] It is produced trough to different mechanisms initiated by hyperviscosity syndrome secondary to extreme polycythemia and cyanosis, which causes an increase in shear stress by passing a high number of red blood cells through the capillary unit in the glomerulus. The subsequent decrease in renal blood flow induce hypoxia, with an angiogenic response mediated by the release of nitric oxide, selective vasodilatation of the afferent arteriole and increased capillary pressure, which causes glomerular hyperfiltration. The reduced blood flow in peritubular capillary also contributes to increase in capillary pressure. [6] Cyanosis triggers an angiogenic stimulus with an increase in the number of capillaries per glomerulus, leading to glomerulomegaly and an increase in the surface area of the glomerular capillary, the podocyte stretches and it is hypertrophied due to stress and there is a damage causing proteinuria – glomerulonephritis due to minimal changes and subsequently to segmental glomerulosclerosis.[6,7]
In our case, we treated him with periodic phlebotomies, angiotensin receptor blocker, anti-platelet, statin and immunosuppressant, but there is still persistent proteinuria. Renal prognosis is unfavorable if the cause is not resolved. The definitive treatment for him is a heart lung transplantation.
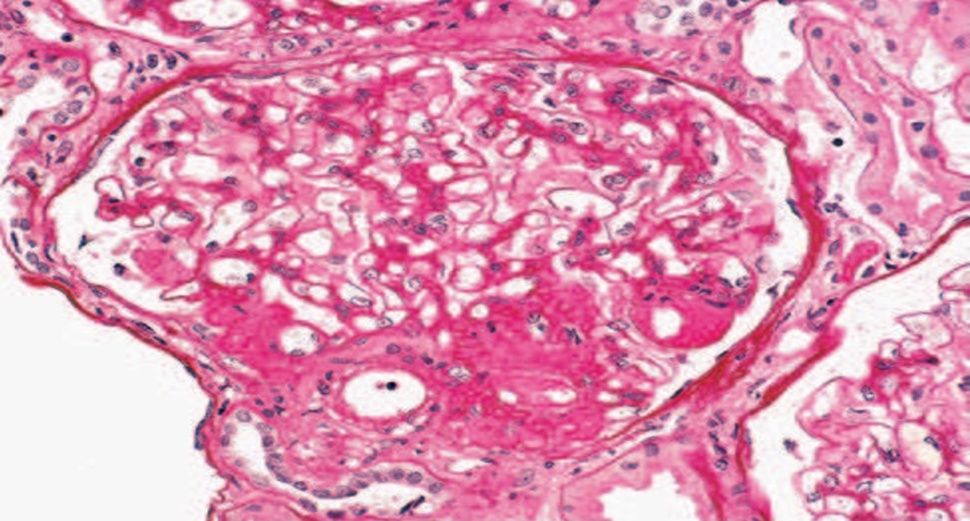
FSGS perihilar variant. A discrete lesion of segmental sclerosis and hyalinosis is located at the glomerular vascular pole (i.e., perihilar). The glomerulus is hypertrophied.
References
- D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis.N Engl J Med. 2011;365:2398–2411.
- Hladunewich MA, Avila-Casada C, Gipson DS. Focal segmental glomerulosclerosis. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Elsevier; 2014.
- Chun MJ, Korbet SM, Schwartz MM, Lewis EJ. FSGS in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol. 2004;15:2169–2217.
- D’Agati VD. The spectrum of focal segmental glomerulosclerosis: new insights. Curr Opin Nephrol Hypertension. 2008;17:271–281.
- Holt T, Filler G. Is it time for a multi-specialty approach to cardio-renal dysfunction in children with cyanotic congenital heart disease? Pediatr Nephrol. 2018;33:359–60.
- Gupte PA, Vaideeswar P, Kandalkar BM. Cyanotic nephropathy– a morphometric analysis. Congenit Heart Dis. 2014;9:280–5.
- Hongsawong N, Khamdee P, Silvilairat S, Chartapisak W. Prevalence and associated factors of renal dysfunction and proteinuria in cyanotic congenital heart disease. Pediatr Nephrol. 2018;33:493–501.
 Dr Yashilha D
Dr Yashilha D