Mr.H a 20 years old male presented to the ER with the history of sudden onset of severe abdominal pain, multiple episodes of vomiting for about 3 days with decreased oral intake and fatigue. Patient was taken to a nearby hospital and diagnosed as Acute Pancreatitis and thence was shifted here for further management. Evaluation at Kauvery hospital, Chennai revealed raised serum lipase, and ongoing pancreatic type abdominal pain at that point (WBC:- 17800, Se.Amylase:- 1130, Se.Lipase:- 1992 and elevated CRP around 250, LDH Levels) CECT Abdomen revealed necrotising Pancreatitis with 40% of pancreatic necrosis and acute fluid collection in the peripancreatic region of size approximately 8cm, Moderate ascites & Bilateral mild pleural effusion, There was no history of alcoholism, abdominal trauma, substance abuse and no evidence of gallstones in the imaging and also the Serum Triglycerides, Serum Calcium and Se.IgG4 were all normal. Patient was observed in ICU for a week and was managed with NPO, IV fluids, Analgesics, antiemetic & PPI, He was started on RT aspiration and was treated with other supportive measures. He developed respiratory distress hence was placed on HFNC in view of SIRS associated respiratory distress. Later he was shifted to ward and was managed for about 2 weeks. He gradually became better and started tolerating oral solids at the time of discharge, With Discharge advice of Low fat diet and pancreatic enzyme replacement therapy.

Image 1:- CECT image showing 40% pancreatic necrosis
He was remaining in follow up after discharge. At 5 weeks from onset of initial pain episode, he reported to the OPD with the complaints of Early satiety, Poor food intake, Anorexia and ongoing weightloss. He was advised to take an MRI abdomen with MRCP which revealed a large pseudocyst of dimensions 20 × 12.8 × 8.9cm compressing the stomach and communicating with the pancreatic duct at mid body of the pancreas. In the view of symptomatic large pseudocyst pancreas it was planned to proceed with EUS guided Cystogastrostomy and ERCP with PD stenting after informed consent from the patient & his parents.

Image 2:- Large Pseudocyst compressing stomach causing Early satiety
(on MRI-T2WI)

Image 3A :- EUS guided Cystogastrostomy 3b:-AXIOS stent Boston scientific
EUS stomach shows a large extraneous impression and a large pancreatic pseudocyst with some necrotic debris. EUS guided puncture using HOT AXIOS was performed and LAMS stent measuring 15mm × 10mm was deployed connecting the pseudocyst with gastric wall along the greater curvature; Around 700 ml of liquefied pancreatic necrosis fluid was drained during the procedure. Post procedure he was monitored in the ICU where he developed fever spikes after 24 hours and was treated with IV antibiotics (Meropenam 1mg Q8H). CT Abdomen was done which confirmed the LAMS in situ and reduction in pseudocyst fluid collection. He was started on oral feeds.
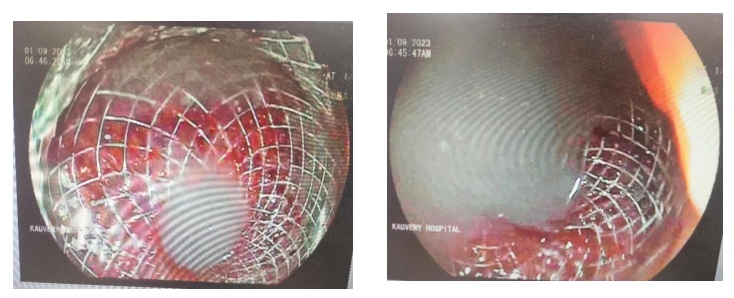
Image 4:- Endoscopy view of deployed LAMS stent and necrotic pancreatic fluid drainage

Image 5:- CT Abdomen – Post LAMS placement
In view of communication of mid body pancreatic duct with Pseudocyst, He then underwent ERCP + PD stenting and sphincterotomy. Guide wire based cannulation of PD was done using the tapered tip sphincterotome, pancreatogram was done which revealed ductal disruption with Disconnected Duct Syndrome. Single pigtail plastic pancreatic stent was deployed into proximal PD slightly crossing the PD leak site. Post procedure was uneventful

Image 6:- Pancreatic Duct leak on ERCP (Disconnected duct syndrome)

Image 7:- ERCP based PD stent placement
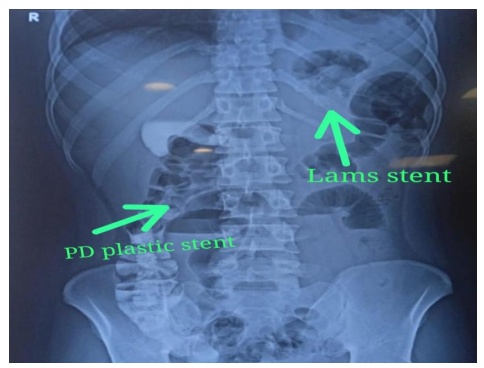
Image 8:- LAMS & PD stents in situ confirmed in Xray Abdomen
He was clinically stable and was able to tolerate feeds orally and improved symptomatically. He was discharged in a stable condition and suggested LAMS Removal after 3 weeks post Cystogastrostomy, followed by PD plastic stent removal.
Case discussion
Lumen apposing metal stents are double flanged fully covered SEMS designed specifically for EUS guided drainage, They are short and have a large diameter for lumen apposing property and reduce the chance of leakage/stent migration (Reference 1) Two types of LAMS are currently popular AXIOS stent Boston scientific corp.,(Natick MA USA) and SPAXUS stent (Taewoong medical, south korea). The HOT AXIOS electrocautery enhanced LAMS allows single step deployment and obviates the need for tract dilatation, Guidewire placement and use of fluoroscopy. EUS with LAMS has yielded high clinical success rates for Pancreatic Pseudo Cyst management in prospective studies. Reference (2-4)
Conclusion
This case illustrates that EUS based interventions are safe, effective and minimize the need for surgery in select cases of Necrotizing Pancreatitis with Large symptomatic pseudocyst.
Reference
1.Bang JY, Varadarajulu S. Lumen-apposing metal stents for endoscopic ultrasonography-guided inter- ventions. Dig Endosc. 2019;31:619-26.
2.Walter D, Will U, Sanchez-Yague A, et al. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid col- lections: a prospective cohort study. Endoscopy. 2015:47:63-7.
3.Song TJ, Lee SS, Moon JH, et al. Efficacy of a novel lumen-apposing metal stent for the treatment of symptomatic pancreatic pseudocysts (with video). Gastrointest Endosc. 2019;90:507-13.
4.Moon JH, Choi HJ, Kim DC, et al. A newly designed fully covered metal stent forlumen apposition in EUS-guided drainage and access: a feasibility study (with videos). Gastrointest Endosc. 2014;79:990-5