Journal scan: A review of 20 recent papers of immediate clinical significance, harvested from major international journals
From the desk of the Editor-in-Chief
(1). Daniel C. Chung et al. Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N Engl J Med 2024;390:973-983.
Abstract
Background
Colorectal cancer is the third most diagnosed cancer in adults in the United States. Early detection could prevent more than 90% of colorectal cancer–related deaths, yet more than one third of the screening-eligible population is not up to date with screening despite multiple available tests. A blood-based test has the potential to improve screening adherence, detect colorectal cancer earlier, and reduce colorectal cancer–related mortality.
Methods
We assessed the performance characteristics of a cell-free DNA (cfDNA) blood-based test in a population eligible for colorectal cancer screening. The coprimary outcomes were sensitivity for colorectal cancer and specificity for advanced neoplasia (colorectal cancer or advanced precancerous lesions) relative to screening colonoscopy. The secondary outcome was sensitivity to detect advanced precancerous lesions.
Results
The clinical validation cohort included 10,258 persons, 7861 of whom met eligibility criteria and were evaluable. A total of 83.1% of the participants with colorectal cancer detected by colonoscopy had a positive cfDNA test and 16.9% had a negative test, which indicates a sensitivity of the cfDNA test for detection of colorectal cancer of 83.1% (95% confidence interval [CI], 72.2 to 90.3). Sensitivity for stage I, II, or III colorectal cancer was 87.5% (95% CI, 75.3 to 94.1), and sensitivity for advanced precancerous lesions was 13.2% (95% CI, 11.3 to 15.3). A total of 89.6% of the participants without any advanced colorectal neoplasia (colorectal cancer or advanced precancerous lesions) identified on colonoscopy had a negative cfDNA blood-based test, whereas 10.4% had a positive cfDNA blood-based test, which indicates a specificity for any advanced neoplasia of 89.6% (95% CI, 88.8 to 90.3). Specificity for negative colonoscopy (no colorectal cancer, advanced precancerous lesions, or nonadvanced precancerous lesions) was 89.9% (95% CI, 89.0 to 90.7).
Conclusions
In an average-risk screening population, this cfDNA blood-based test had 83% sensitivity for colorectal cancer, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions
(2). Imperiale TF, Porter K, Zella J, et al. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med. 2024;390(11):984-993.
Abstract
Background
A next-generation multitarget stool DNA test, including assessments of DNA molecular markers and hemoglobin level, was developed to improve the performance of colorectal cancer screening, primarily with regard to specificity.
Methods
In a prospective study, we evaluated a next-generation multitarget stool DNA test in asymptomatic adults 40 years of age or older who were undergoing screening colonoscopy. The primary outcomes were sensitivity of the test for colorectal cancer and specificity for advanced neoplasia (colorectal cancer or advanced precancerous lesions). Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. Secondary objectives included the quantification of sensitivity for advanced precancerous lesions and specificity for nonneoplastic findings or negative colonoscopy and comparison of sensitivities for colorectal cancer and advanced precancerous lesions between the multitarget stool DNA test and a commercially available fecal immunochemical test (FIT).
Results
Of 20,176 participants, 98 had colorectal cancer, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy. With the next-generation test, sensitivity for colorectal cancer was 93.9% (95% confidence interval [CI], 87.1 to 97.7), and specificity for advanced neoplasia was 90.6% (95% CI, 90.1 to 91.0). Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3 to 45.6), and specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2 to 93.1). With the FIT, sensitivity was 67.3% (95% CI, 57.1 to 76.5) for colorectal cancer and 23.3% (95% CI, 21.5 to 25.2) for advanced precancerous lesions; specificity was 94.8% (95% CI, 94.4 to 95.1) for advanced neoplasia and 95.7% (95% CI, 95.3 to 96.1) for nonneoplastic findings or negative colonoscopy. As compared with FIT, the next-generation test had superior sensitivity for colorectal cancer (P<0.001) and for advanced precancerous lesions (P<0.001) but had lower specificity for advanced neoplasia (P<0.001). No adverse events occurred.
Conclusions
The next-generation multitarget stool DNA test showed higher sensitivity for colorectal cancer and advanced precancerous lesions than FIT but also showed lower specificity.
(4). Bryan D. Choi et al, Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. NEJM. March 13, 2024, DOI: 10.1056/NEJMoa2314390
Summary
In this first-in-human, investigator-initiated, open-label study, three participants with recurrent glioblastoma were treated with CARv3-TEAM-E T cells, which are chimeric antigen receptor (CAR) T cells engineered to target the epidermal growth factor receptor (EGFR) variant III tumor-specific antigen, as well as the wild-type EGFR protein, through secretion of a T-cell–engaging antibody molecule (TEAM). Treatment with CARv3-TEAM-E T cells did not result in adverse events greater than grade 3 or dose-limiting toxic effects. Radiographic tumor regression was dramatic and rapid, occurring within days after receipt of a single intraventricular infusion, but the responses were transient in two of the three participants.
(5). Bao-ping Xu et al, Green Dialysis Effluent and Plasma. March 14, 2024,N Engl J Med 2024; 390:e27

A 72-year-old man with end-stage kidney disease who began receiving continuous renal-replacement therapy in the intensive care unit (ICU) was noted to have green-colored dialysis effluent and plasma. Seven hours earlier, the patient had presented to the emergency department with a gastrointestinal perforation. An exploratory laparotomy was performed, and a gastric perforation caused by a previously unknown gastric adenocarcinoma was identified. A 40-ml solution of 0.1% methylene blue was injected into the tissue surrounding the gastric tumor to identify the lymph nodes draining the region. The gastric tumor and 11 lymph nodes were subsequently excised. Postoperatively, the patient was admitted to the ICU for the treatment of septic shock. Four hours after the initiation of continuous renal-replacement therapy, the green color was noted in dialysis effluent (Panel A) and in plasma (Panel B) that had been obtained from the patient. A diagnosis of fluid discoloration owing to the intraoperative use of methylene blue was made. Methylene blue may discolor skin or body fluids as a benign side effect of its use. Twenty-four hours after the initiation of continuous renal-replacement therapy, the green discoloration had abated. After a 1-month hospital stay, the patient was discharged home. Immunotherapy was later initiated for the treatment of liver metastases.
(6). Samantha Anderer. Stem Cell Transplants Might Boost Lung Function in People With COPD. JAMA. March 13, 2024.
An early clinical trial involving 20 participants in China suggests that pulmonary stem cell transplants could benefit patients with chronic obstructive pulmonary disease (COPD). The participants had stage 2 to stage 4 COPD and were between the ages of 40 and 75 years.
The researchers took bronchial cells known as P63+ stem cells from participants and cultured them over 3 to 5 weeks before transplanting them back into the lungs. There were minimal adverse events, and after 24 weeks, gas transfer capacity increased 18% in the transplant group and decreased 17% in the control group. In addition, the participants who received transplants had more than a 30-meter increase in 6-minute walking distance.
Because COPD causes irreversible lung damage, there is a need for treatments that regenerate lung cells, the researchers noted in Science Translational Medicine. Despite the small sample size of patients, who were all male, the findings “demonstrated the feasibility and safety” of P63+ lung stem cell transplants in people with COPD, they added.
(7). Simon Dadoun et al. Congenital toxoplasmosis of the brain caused by infection in late pregnancy. March 16, 2024. DOI:https://doi.org/10.1016/S0140-6736(24)00257-5.
A fetus, on routine growth ultrasound scan, at 36 weeks, was found to have severely enlarged lateral ventricles and a dilated third ventricle, and normal growth (figure). The findings were confirmed by MRI (figure), which also showed signal abnormalities in the caudothalamic grooves, and extensive signals in the periventricular white matter indicating possible oedema, macrocephaly, and hepatosplenomegaly.

Figure: In utero and congenital toxoplasmosis of the brain caused by infection in late pregnancy
The mother, a 33-year-old primigravida, had conceived by in-vitro fertilisation; both parents were carriers for cystic fibrosis, and preimplantation genetic testing of the embryo was negative for aneuploidy and mutations in CFTR, the gene encoding the epithelial ion channel. No family history of birth defects, intellectual impairment, genetic disorders, or congenital anomalies was recorded. The previous second-trimester anatomy scan had shown typical growth and no fetal anomalies; and these new findings raised suspicion for a congenitally acquired infection.
Further discussion with the mother found that she had no known exposure to Toxoplasma gondii or anyone with toxoplasmosis, but she did report proximity to farm animals, feral cat exposure in the backyard of her home, and ingestion of cooked wild venison multiple times during the pregnancy.
Maternal TORCH (T gondii, cytomegalovirus, rubella virus, and herpes simplex virus) titres found elevated IgG and IgM titres for T gondii (normal range <7·2 IU/mL and less than arbitrary units per mL, respectively).
Given the late gestational age of the fetus, amniocentesis was deferred and, on discussion with the mother, it was decided to deliver the baby; an uncomplicated elective caesarean section delivered, at 38 weeks’ gestational age, a 3410 g neonate (41st percentile for gestational age). Apgar scores were 8 and 9 (typical range: between 7 and 10). Maternal and fetal cord blood samples were collected and sent to a toxoplasmosis reference laboratory. Results confirmed maternal toxoplasmosis: T gondii IgG Sabin-Feldman dye test positive 1:8000 (normal <1:16), low IgG avidity testing, T gondii IgM ELISA positive 10·0 (normal <2·0), AC/HS 400/400, IgA ELISA positive, and IgE ELISA positive, indicating a diagnosis of an acute infection with T gondii acquired during the third trimester of pregnancy.
Examination of the placenta was notable for chronic villous inflammation with multiple cysts identified within the chorionic villi and membranes with positive immunohistochemical stains for T gondii.
At delivery, the newborn presented with diffuse hypotonia. Neonatal blood samples showed T gondii IgG Sabin-Feldman dye test 1:8000 positive, T gondii IgM ELISA immunosorbent agglutination assay positive, and T gondii IgA positive. T gondii PCR on whole blood and cerebrospinal fluid were also positive, confirming congenital toxoplasmosis.
Neonatal brain MRIs showed severe dilatation bilaterally of the lateral and third ventricules, cortical thinning, aqueductal stenosis, and bilateral periventricular and basal ganglia calcifications. Ocular involvement was also noted with bilateral peripheral retinal lesions and vitritis. The infant was admitted to our neonatal intensive care unit for 45 days receiving pyrimethamine and sulfadiazine with folinic acid, steroids, supportive measures, and feeding therapy. Due to symptomatic hydrocephalus, a ventriculoperitoneal shunt was put in place after 35 days of life.
At follow-up 6 months later, the infant was recovering well, still on antiparasitic therapy with the ventriculoperitoneal shunt in place; he was meeting his milestones appropriately, although there was a concern for congenital palsy of the right upper arm.
Toxoplasmosis is an endemic disease caused by T gondii and an estimated 170 infants are born with congenital toxoplasmosis yearly in the USA. The protozoan infection is transmitted to humans by ingesting undercooked meats containing the parasite’s cysts, contaminated vegetables, or by contact with cat faeces. Primary maternal infection is asymptomatic in about two-thirds of patients. When symptomatic, it typically presents with mild malaise, headache, and lymphadenopathies. The overall risk for congenital toxoplasmosis following maternal primary infection ranges from 20% to 50% without treatment. The risk of transmission increases with gestational age and can be as high as 60% if acquired by greater than 36 weeks of gestation; the earlier the infection is, the more severe the impact on the fetus. Even though most infected infants do not have clinical signs of infection at birth, up to 90% will develop sequelae later in life. Diagnosis of maternal toxoplasmosis is usually confirmed serologically. Given the high false-positive rates of commercially available IgM-based testing kits, confirmatory testing at a reference laboratory should always be done.
We were all involved in providing care for the patient and his mother, and in reviewing and editing the manuscript. SD wrote the original draft and administered the project. MSC conceptualised the project and supervised the case. Written consent for publication was obtained from the baby’s mother.
(8). Mitsuhide Naruse et al. Targeted molecular medicine: advances in the treatment of metastatic phaeochromocytoma and paraganglioma. February 22, 2024DOI:https://doi.org/10.1016/S0140-6736(23)02828-3.
WHO defines phaeochromocytomas and paragangliomas as neuroendocrine tumours arising from chromaffin cells of the adrenal medulla or extra-adrenal paraganglia, respectively. Since catecholamine-secreting phaeochromocytomas and paragangliomas were first successfully resected 100 years ago, major advances have occurred in the measurement of catecholamine and catecholamine metabolites, computed cross-sectional imaging, total body nuclear imaging, and laparoscopic approaches to tumour resection. In the early 2000s, many phaeochromocytoma and paraganglioma susceptibility genes were reported, underscoring the frequent hereditary nature of these rare endocrine neoplasms. Concomitantly, metastatic phaeochromocytomas and paragangliomas have been increasingly recognised to be first detected up to 50 years postoperatively. In 2017, WHO’s classification of tumours of endocrine organs advised that all phaeochromocytomas and paragangliomas have malignant potential—a potential that is only confirmed when metastatic disease is documented. In the 21st century, the primary diagnostic challenges for phaeochromocytoma and paraganglioma have become the early distinction between metastatic and non-metastatic forms and detecting pathogenic variants in the genes that predispose to the development of phaeochromocytomas and paragangliomas and distant metastases. On the management front, the main challenges remain the detection and treatment of metastatic phaeochromocytoma and paraganglioma, for which curative options are yet to be established.
(9). Richard Horton. What have we done to medical students?. The Lancet. March 16, 2024. DOI: https://doi.org/10.1016/S0140-6736(24)00526-9.
“We are not cherished.” Those words still outrage me 20 years on. I was part of a roadshow on medical professionalism touring universities in England to discuss Doctors in Society, a report from the Royal College of Physicians. Baroness Julia Cumberlege chaired the working party and I was her scribe. Our goal was to advance a new conception of medical professionalism, one based less on authority and more on partnership. We were passionate about what medicine was and what it could achieve—for patients and for society. At one medical school, we took part in a debate about the future of our profession with an audience of students and young doctors. Far from being enthusiastic about their new careers, they were despondent. And that is when one made the remark about not being cherished. She was comparing her experiences with those of her friends who had pursued other careers—law, accountancy, architecture. They all felt valued in their new workplaces. They were welcomed and made to feel important members of a team. Their contributions, skills, and efforts were recognised, appreciated, and respected. Their environments made them feel engaged and motivated. In medicine, by contrast, these students felt invisible and taken for granted. Nobody seemed to care about their work or their experiences. They felt abandoned in a system that simply did not care. Our modern vision for 21st-century medical professionalism meant little if we were ignoring the circumstances and feelings of these newly qualified colleagues. What kind of profession had we created?
So, when Dinesh Bhugra stood in front of an audience at the Royal College of Psychiatrists (he is a former President) last week and asked, “What are we doing to these bright young people?”, it seemed we were guilty of extraordinary negligence. He was speaking at the launch of his compelling, indeed shocking, new book, The Mental Health of Medical Students: Supporting Wellbeing in Medical Education. Every doctor who interacts with medical students should read this book. Sarah Farrell, a neurosurgeon in Oxford and a co-editor with Bhugra and Andrew Molodynski, put the problem succinctly: “You shouldn’t have to destroy your own mental health in order to help others.” As Debbie Cohen and Thomas Kitchen write: “medical education across the globe is a failing system. It is failing because it is propping up failing healthcare systems. Both systems need regeneration.” Too many students (between 60% and 99%) are burnt out, exhausted, depressed, and distressed. Bhugra and his team have produced a Medical Student Wellbeing Charter as a foundation for revitalising the experiences of students. I asked one student I know if her medical school fulfilled any of the eleven “asks” in the Charter. “I’m not entirely sure if we fulfil a single one of these, to be honest”, she wrote.
There are few things more infuriating than listening to those who say, “It was better in my day.” Of course, it wasn’t. Working on-call continuously from Friday until Monday was not good for any patient. Lying in the bath while your crash bleep went off was not high-quality medical care. Smoking next to the operating room, waiting for the next patient to be wheeled in, was not setting a good example. 40 years ago, the treatments available for life-threatening conditions were rudimentary compared with today’s standards. But I think we did feel, if not cherished, then at least wanted and looked after. We had year cohorts of a size that did not make you feel a tiny cog in a vast depersonalised machine. We had small group tutorials that encouraged discussion and dissent. You could pursue particular interests with a faculty that welcomed your curiosity. You had time to read and to think. And when you were on the wards, you were part of a team—a “firm”—that guided you professionally and pastorally as your experiences in medicine evolved and matured. In 2024, it is surely time to end the pervasive harm medical education is inflicting on its students. Medical schools are presiding over an epidemic of injury within their student body. Deans of medicine seem oblivious or indifferent. What have we done to the most precious opportunity medicine possesses—each new generation of medical students? Dinesh Bhugra, Sarah Farrell, and Andrew Molodynski have exposed a scandal in plain sight at the heart of medicine. Who will act?
(10). Cristian-Mihail Niculae et al. Rhino-orbital-cerebral Mucormycosis. N Engl J Med. 2024; 390:e30

An 82-year-old man with type 2 diabetes presented to the hospital with a 2-week history of headache and spontaneous bruising around his right eye.
Six weeks before presentation, he had been hospitalized for severe coronavirus disease 2019 pneumonia and received treatment with high-dose glucocorticoids. He had continued to receive a tapering dose of glucocorticoids for immune thrombocytopenia in the 4 weeks since hospital discharge.
On physical examination, ecchymosis was seen over the upper eyelid (Panel A), and a black eschar was present in the right naris (arrow). The patient also had proptosis, ophthalmoplegia, and vision loss in the right eye. Nasal endoscopy showed necrotic-appearing black eschars within the nasal cavity and perforation of the nasal septum (Panel B and video).
Owing to concern for mucormycosis, antifungal therapy was initiated. Magnetic resonance imaging of the head showed involvement of the right orbit, the maxillary and ethmoid sinuses on both sides, and the right frontal lobe of the brain (Panel C, asterisk). Histopathological findings in a biopsy specimen from the right maxillary sinus were consistent with invasive mucormycosis.
A diagnosis of rhino-orbital-cerebral mucormycosis was made. The patient was transferred to a tertiary care hospital, where extensive surgical débridement was performed. He died in the hospital 10 weeks after presentation.
(11). Margarita Safir et al. Dry eye disease management. BMJ 2024;384:e077344
What you need to know
Dry eye disease is a highly prevalent chronic ocular condition
The mainstays of dry eye disease management include lifestyle modification, eyelid hygiene, and lubrication
Novel therapeutic methods using intense pulse light or thermal pulsation may offer future benefit to patients with this condition
Dry eye disease is common, with large cross sectional studies estimating a prevalence of 19-31% among the adult population123 and 6-23% among children.
This condition is often associated with ocular discomfort and visual symptoms, and severity can range from mild occasional discomfort to sight threatening disease. This article offers an approach to identifying and managing dry eye disease and discusses novel treatment modalities.
What causes dry eye disease?
Adequate lubrication of the ocular surface requires appropriate coverage of the ocular surface by the eyelids and sufficient production of tear film components, including both the aqueous component (produced by the lacrimal and accessory glands) and the lipid component (produced by the meibomian glands and the conjunctival mucin-producing glands). Damage to the ocular surface can induce an inflammatory response causing further ocular surface damage and propagating disease development. While meibomian gland dysfunction is the most common cause of dry eye disease, the aetiology of dry eyes is often multifactorial. Additional common causes of dry eye disease include blepharitis, rosacea, commonly prescribed drugs, and environmental factors.
Medical Conditions That are Associated With or Aggravate Dry Eye Disease
Demographic: Older age, Female
Medications: Antihistamine, Antidepressants, β blockers, Retinoic acid, Hormone replacement therapy
Environmental factors and lifestyle: Smoking, Low humidity, Air conditioning or heating systems, Exposure to dust, allergens, air pollution, Prolonged digital device use, Reduced sleep duration
Ocular conditions: Contact lens use, Ocular surgery, Trauma to ocular surface, Blepharitis, Thyroid eye disease, Previous herpetic keratitis
Systemic conditions: Rosacea, Sjogren’s syndrome, Rheumatoid arthritis, Systemic lupus erythematosus, Graft versus host disease, Stevens-Johnson syndrome, Vitamin A deficiency, Diabetes, Parkinson’s disease, Multiple sclerosis
(12). Zachary Nash, clinical research fellow et al. Premature ovarian insufficiency. BMJ 2024;384:e077469.
What you need to know
Diagnosis of premature ovarian insufficiency (POI) is based on menstrual disturbance and raised follicle stimulating hormone in women under 40. Untreated POI affects quality of life and increases long term risk of cardiovascular disease, osteoporosis and fracture, and slightly reduces life expectancy. Women with POI should receive hormone therapy and lifestyle advice.
POI is associated with infertility, and egg donation may be needed. Refer women with a diagnosis of POI to a specialist service to manage their physical, psychological, and reproductive health
Premature ovarian insufficiency (POI) is defined as menopause before the age of 40 (two standard deviations below the average age of menopause). It is also known as premature menopause or primary ovarian insufficiency, but POI is the preferred term.1 Premature is preferred to primary to avoid confusion with primary amenorrhoea and to highlight the age of onset. Insufficiency is preferred to failure to reflect possible fluctuation in ovarian function and to avoid negative connotations.
The prevalence of POI has historically been estimated at 1-3% in high income countries based on data from cohort studies.45 A recent meta-analysis of 31 studies estimated the global prevalence of POI to be 3.7% (95% confidence interval (CI) 3.1% to 4.3%) with a higher prevalence in middle and low income countries.
POI affects wellbeing, fertility, and if untreated is associated with lower bone mineral density (BMD) and increased risk of osteoporosis, cardiovascular disease, possibly dementia (in women with POI after surgical menopause)11 as well as overall increased all-cause mortality
(13). New wave of potential therapies would bring shift in treatment paradigm for COPD
The current gap in the COPD armamentarium is the absence of biologics. Dupixent (Sanofi/Regeneron), an interleukin-4 and interleukin-13 inhibitor, has the potential to address this gap as positive results were reported from two phase III clinical trials, BOREAS and NOTUS, and in both trails, the primary endpoint of the trials was met.
The trails show that dupixent significantly reduced moderate or severe acute COPD exacerbations by 30 percent in the BOREAS trail and 34% in the NOTUS trail over 52% and improved lung function at 12 weeks that was sustained through 52 weeks, compared to placebo.
The recent submission of clinical trial data on Dupixent and Ensifentrine to the food and drug administration (FDA) indicates the entry of new therapies for chronic obstructive pulmonary disease (COPD) in 2024. Phase III trails were able to meet endpoints and used as a maintenance or add on therapies to the standard of care.
The standard of care when treating COPD patients involves the use of either short or long acting beta-2 agonists or muscarinic antagonist as a monotherapy or in combination. Alternatively, for exacerbators, the addition of inhaled corticosteroids as triple therapy regimen or taken a long acting beta agonist as a fixed dose combination. Phosphodiesterase inhibitors (PDE) can be used as an add on to triple therapy, depending on the severity of COPD. These therapies are tolerable by patients; however, the progressive nature of COPD has created a need for maintenance therapy.
A first in- class dual PDE3/4 Inhibitors administered as a nebulised formulation, has also gained the FDAs interest after two positive phase III trails (ENHANCE-1 and ENHANCE -2) met their primary endpoint of reducing exacerbations and improving lung function.
This indicates a shift in the treatment paradigm for COPD, with the introduction of biologics and a novel first-in-class dual phosphodiesterase inhibitor, given their positive clinical trail data.
(14). Cholesterol Treatment Trialists’ (CTT) Collaboration. Effects of statin therapy on diagnoses of new-onset diabetes and worsening glycaemia in large-scale randomised blinded statin trials: an individual participant data meta-analysis. DOI:https://doi.org/10.1016/S2213-8587(24)00040-8
Summary
Background
Previous meta-analyses of summary data from randomised controlled trials have shown that statin therapy increases the risk of diabetes, but less is known about the size or timing of this effect, or who is at greatest risk. We aimed to address these gaps in knowledge through analysis of individual participant data from large, long-term, randomised, double-blind trials of statin therapy.
Methods
We conducted a meta-analysis of individual participant data from randomised controlled trials of statin therapy that participated in the CTT Collaboration. All double-blind randomised controlled trials of statin therapy of at least 2 years’ scheduled duration and with at least 1000 participants were eligible for inclusion in this meta-analysis. All recorded diabetes-related adverse events, treatments, and measures of glycaemia were sought from eligible trials. Meta-analyses assessed the effects of allocation to statin therapy on new-onset diabetes (defined by diabetes-related adverse events, use of new glucose-lowering medications, glucose concentrations, or HbA1c values) and on worsening glycaemia in people with diabetes (defined by complications of glucose control, increased use of glucose-lowering medication, or HbA1c increase of ≥0·5%). Standard inverse-variance-weighted meta-analyses of the effects on these outcomes were conducted according to a prespecified protocol.
Findings
Of the trials participating in the CTT Collaboration, 19 trials compared statin versus placebo (123 940 participants, 25 701 [21%] with diabetes; median follow-up of 4·3 years), and four trials compared more versus less intensive statin therapy (30 724 participants, 5340 [17%] with diabetes, median follow-up of 4·9 years). Compared with placebo, allocation to low-intensity or moderate-intensity statin therapy resulted in a 10% proportional increase in new-onset diabetes (2420 of 39 179 participants assigned to receive a statin [1·3% per year] vs 2214 of 39 266 participants assigned to receive placebo [1·2% per year]; rate ratio [RR] 1·10, 95% CI 1·04–1·16), and allocation to high-intensity statin therapy resulted in a 36% proportional increase (1221 of 9935 participants assigned to receive a statin [4·8% per year] vs 905 of 9859 participants assigned to receive placebo [3·5% per year]; 1·36, 1·25–1·48). For each trial, the rate of new-onset diabetes among participants allocated to receive placebo depended mostly on the proportion of participants who had at least one follow-up HbA1c measurement; this proportion was much higher in the high-intensity than the low-intensity or moderate-intensity trials. Consequently, the main determinant of the magnitude of the absolute excesses in the two types of trial was the extent of HbA1c measurement rather than the proportional increase in risk associated with statin therapy. In participants without baseline diabetes, mean glucose increased by 0·04 mmol/L with both low-intensity or moderate-intensity (95% CI 0·03–0·05) and high-intensity statins (0·02–0·06), and mean HbA1c increased by 0·06% (0·00–0·12) with low-intensity or moderate-intensity statins and 0·08% (0·07–0·09) with high-intensity statins. Among those with a baseline measure of glycaemia, approximately 62% of new-onset diabetes cases were among participants who were already in the top quarter of the baseline distribution. The relative effects of statin therapy on new-onset diabetes were similar among different types of participants and over time. Among participants with baseline diabetes, the RRs for worsening glycaemia were 1·10 (1·06–1·14) for low-intensity or moderate-intensity statin therapy and 1·24 (1·06–1·44) for high-intensity statin therapy compared with placebo.
Interpretation
Statins cause a moderate dose-dependent increase in new diagnoses of diabetes that is consistent with a small upwards shift in glycaemia, with the majority of new diagnoses of diabetes occurring in people with baseline glycaemic markers that are close to the diagnostic threshold for diabetes. Importantly, however, any theoretical adverse effects of statins on cardiovascular risk that might arise from these small increases in glycaemia (or, indeed, from any other mechanism) are already accounted for in the overall reduction in cardiovascular risk that is seen with statin therapy in these trials. These findings should further inform clinical guidelines regarding clinical management of people taking statin therapy.
Funding
British Heart Foundation, UK Medical Research Council, and Australian National Health and Medical Research Council.
(15). Valvular heart disease, the Lancet series. March 27, 2024
Executive Summary
Valvular heart disease (VHD) is becoming more prevalent in an ageing population, leading to challenges in diagnosis and management. This two-part Series offers a comprehensive review of changing concepts in VHD, covering diagnosis, intervention timing, novel management strategies, and the current state of research.
The first paper highlights the remarkable progress made in imaging and transcatheter techniques, effectively addressing the treatment paradox wherein populations at the highest risk of VHD often receive the least treatment. These advances have attracted the attention of clinicians, researchers, engineers, device manufacturers, and investors, leading to the exploration and proposal of treatment approaches grounded in pathophysiology and multidisciplinary strategies for VHD management. The second article focuses on future innovations, including computational, pharmacological, and bioengineering approaches. These include integrating artificial intelligence and imaging, novel pharmacological strategies, and engineered heart valve tissue. Together, these articles emphasise the importance of early detection, personalised management, and cutting-edge interventions to optimise outcomes amid the evolving landscape of VHD.
Praz et al.Valvular heart disease: from mechanisms to management. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)02755-1/abstract
Summary
Valvular heart disease is common and its prevalence is rapidly increasing worldwide. Effective medical therapies are insufficient and treatment was historically limited to the surgical techniques of valve repair or replacement, resulting in systematic underprovision of care to older patients and those with substantial comorbidities, frailty, or left ventricular dysfunction. Advances in imaging and surgical techniques over the past 20 years have transformed the management of valvular heart disease. Better understanding of the mechanisms and causes of disease and an increasingly extensive and robust evidence base provide a platform for the delivery of individualised treatment by multidisciplinary heart teams working within networks of diagnostic facilities and specialist heart valve centres. In this Series paper, we aim to provide an overview of the current and future management of valvular heart disease and propose treatment approaches based on an understanding of the underlying pathophysiology and the application of multidisciplinary treatment strategies to individual patients.
Sengupta et al. The future of valvular heart disease assessment and therapy
Summary
Valvular heart disease (VHD) is becoming more prevalent in an ageing population, leading to challenges in diagnosis and management. This two-part Series offers a comprehensive review of changing concepts in VHD, covering diagnosis, intervention timing, novel management strategies, and the current state of research. The first paper highlights the remarkable progress made in imaging and transcatheter techniques, effectively addressing the treatment paradox wherein populations at the highest risk of VHD often receive the least treatment. These advances have attracted the attention of clinicians, researchers, engineers, device manufacturers, and investors, leading to the exploration and proposal of treatment approaches grounded in pathophysiology and multidisciplinary strategies for VHD management. This Series paper focuses on innovations involving computational, pharmacological, and bioengineering approaches that are transforming the diagnosis and management of patients with VHD. Artificial intelligence and digital methods are enhancing screening, diagnosis, and planning procedures, and the integration of imaging and clinical data is improving the classification of VHD severity. The emergence of artificial intelligence techniques, including so-called digital twins—eg, computer-generated replicas of the heart—is aiding the development of new strategies for enhanced risk stratification, prognostication, and individualised therapeutic targeting. Various new molecular targets and novel pharmacological strategies are being developed, including multiomics—ie, analytical methods used to integrate complex biological big data to find novel pathways to halt the progression of VHD. In addition, efforts have been undertaken to engineer heart valve tissue and provide a living valve conduit capable of growth and biological integration. Overall, these advances emphasise the importance of early detection, personalised management, and cutting-edge interventions to optimise outcomes amid the evolving landscape of VHD. Although several challenges must be overcome, these breakthroughs represent opportunities to advance patient-centred investigations.
Hahn et al. Heart valve disease: at the threshold of a new era in patient management. The LancetPublished: March 27, 2024
Valvular heart disease (VHD) represents a range of different disease states and is a major public health problem that is only set to increase with an ageing population.
For many decades, the only available treatment was open-heart surgery to repair or replace the valve once patients develop severe symptomatic disease. However, assessing symptomatic status in older patients with VHD is challenging, and these patients are often not well suited to major heart surgery. The recent development of transcatheter aortic valve intervention and other percutaneous treatment options has revolutionised how patients with VHD are now treated and has reinvigorated scientific research in this area. Indeed, VHD has become an area of intense innovation and rapid development that appears set to further improve how these conditions are assessed and treated worldwide. Two Series papers on VHD in The Lancet address some of these advances.
The first Series paper, on mechanisms and management of VHD,
recognises that the paradox whereby populations at the greatest risk were least likely to receive treatment has been addressed in recent years by advances in imaging and the rapid emergence of transcatheter techniques. This Series paper provides an overview of the current management of VHD and treatment approaches based on an understanding of the underlying pathophysiology and application of multidisciplinary treatment strategies to individual patients.
This Lancet Series on heart valve disease is timely, given that novel approaches are urgently required. Recent natural history studies show the persistent failure of modern medicine to promptly diagnose and treat VHD, highlighting that patients with VHD remain limited by an adverse long-term prognosis that persists even after valve intervention. Indeed, compared with patients without VHD, the risk of all-cause death is 25% higher in patients with mitral VHD, 60% higher in patients with aortic VHD, and more than 250% higher in those with tricuspid regurgitation.
Recent developments in imaging have played a key role in refining our thinking by allowing the earlier detection of the myocardial structural changes that accompany VHD.
Patients with regurgitant lesions develop ventricular dilatation, fibrosis, and, ultimately, systolic dysfunction, which, if left too late, will not reverse following valve intervention. Meanwhile, patients with aortic stenosis develop a hypertrophic response that will eventually decompensate, leaving patients with irreversible myocardial scarring that drives the development of both systolic and diastolic dysfunction. In both scenarios, the irreversible structural changes that accrue in the myocardium while patients await valve intervention translate into an increased risk of heart failure and death that persists long after the valve disease has been treated.
Waiting for severe symptomatic valve disease might, therefore, be leaving things too late; instead, it might be preferable to intervene at an earlier stage, when the myocardium is still healthy.
Novel imaging modalities, such as myocardial strain imaging by echocardiography and detection of interstitial (extracellular) expansion or fibrosis and loss of myofibers using cardiac MRI, provide more sensitive markers of myocardial health that might predict future adverse events and the need for early valve replacement.
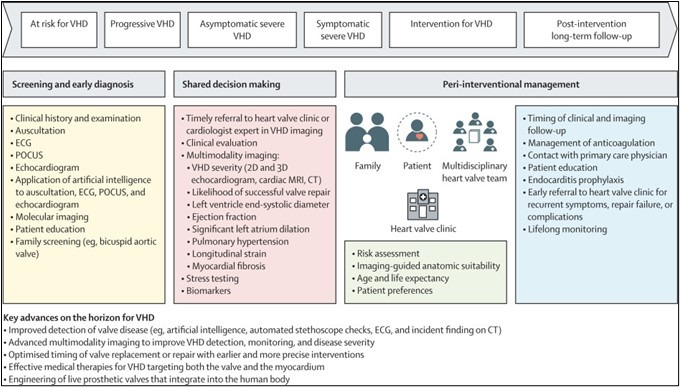
Changing paradigm of valvular heart disease management with key potential advances
Nonetheless, early intervention is not without its own potential risks—in particular, the premature exposure of patients to the problems associated with valve replacement. These problems include endocarditis and bleeding in patients with mechanical valves who require anticoagulation and valve degeneration in patients with bioprosthetic valves. On average, bioprosthetic valves last between 10 years and 15 years before they calcify, stenose, leak, and fail. Prematurely timed valve implantation, therefore, increases the risk of patients requiring a higher-risk redo procedure later in life. Thus, when considering the optimal timing for VHD intervention, multiple competing factors need to be balanced.
Important evidence gaps remain regarding specific medical therapy, the optimal timing of surgical and transcatheter interventions, and optimal management following interventions. Ultimately, randomised controlled trials are required to establish when best to intervene in different types of valve disease. Ideally, such trials will incorporate long-term follow-up, so that the risks and benefits of early intervention are fully captured. Two small randomised controlled trials, in 2020 and 2022, indicated that early intervention in asymptomatic patients with severe or critical aortic stenosis reduces the risk of death or admission with heart failure after 2–4 years, compared with the current watchful waiting approach. Larger ongoing trials will report later this year and, if also positive, might well alter current clinical guidelines.
Other studies are extending this rationale yet further and investigating whether valve intervention should be considered in patients with moderate disease who have either overt left ventricular systolic impairment or more subtle imaging markers or myocardial damage.
The second Series paper, on the future of VHD assessment and therapy, focuses on innovations revolving around computational, pharmacological, and bioengineering approaches that are transforming how we diagnose and manage patients with VHD. This Series paper discusses four key areas where progress is being made: artificial intelligence and digital methods to enhance screening, diagnosis, and planning procedures; the integration of imaging and clinical data to improve the classification and risk stratification of VHD severity; the development of medical therapies, molecular targets, and novel pharmacological strategies to halt the progression of VHD; and advances in efforts to engineer heart valve tissue. Despite these advances, there are barriers to the adoption or development of novel methods of detection, risk stratification, and treatment of VHD. These barriers include privacy issues, under-representation of populations at risk (creating bias), and requirements for personalised or precision genomics to guide therapy.
Together, these Lancet Series papers highlight the major advances that lie on the horizon and underscore the considerable innovation and momentum driving this field. The prospect of pharmacological modification of VHD, in particular, would seem a realistic and long-awaited goal.
We are excited to see where these advances lead over both the short and long terms, including the new evidence-based treatment strategies that we will be able to offer to our patients.
(16). Ole Haagen Nielsen, John Mark Gubatan, Kaija-Leena Kolho, Sarah Elizabeth Streett, Cynthia Maxwell. Updates on the management of inflammatory bowel disease from periconception to pregnancy and lactation. The Lancet. 2024;403 (10433):P1291-1303.
Summary
Inflammatory bowel disease (IBD) affects reproductive planning due to psychological effects and mechanical problems related to surgery. Children of people with IBD have an increased risk of about 10% if one parent has IBD and up to 33% if both parents have IBD. The fertility of people with IBD is similar to the general population, but fertility might be reduced in individuals with active IBD, ileal pouch-anal anastomosis, or perianal Crohn’s disease. Flaring disease during pregnancy increases complications, such as preterm birth. Thus, disease management with appropriate medications can optimize outcomes. As most medications have minimal fetal risks, people with IBD should be informed about the risks of stopping medications and the importance of maintaining remission. A period of disease remission is advisable before pregnancy and could reduce the risks for both the pregnant person and the fetus. Flexible endoscopy, intestinal ultrasound, and gadolinium-free magnetic resonance enterography are safe during pregnancy. We provide state-of-the-art knowledge on the basis of the latest evidence to ensure successful pregnancy outcomes in controlled IBD.
(17). Guangyao Cai, Artificial intelligence-based models enabling accurate diagnosis of ovarian cancer using laboratory tests in China: a multicentre, retrospective cohort study. The Lancet. 2024;6 (3):176-186.
A 75-year-old woman who attended for a routine x-ray appointment was given the all-clear after the image was reported as having no atypical findings (figure). The investigation had been arranged as part of the follow-up package for the patient who, 3 years earlier, had an abdominoperineal resection for rectal and anal cancer. The rectal carcinoma was a moderately to well differentiated tubular adenocarcinoma, 25 mm × 20 mm, pT2N0M0 stage I; the anal carcinoma was a squamous cell carcinoma, 5 mm × 5 mm, pTisN0M0 stage 0.
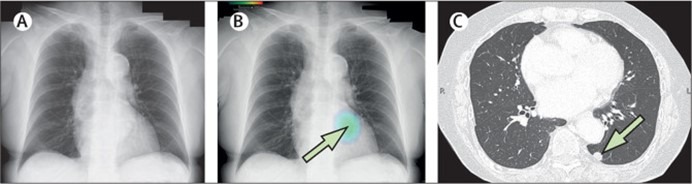
The x-ray was then examined by an artificial intelligence (AI)-based radiodiagnosis package, which was developed by CXR-AID (Fujifilm Medical Corporation, Tokyo, Japan) and which was adopted for routine use of all chest x-rays in our hospital last year; the AI report indicated a left lower lobe nodule (figure).
On examination the patient was generally well; a chest examination found no abnormalities.
Laboratory investigations—including a complete blood count, serum C-reactive protein concentration, and serum cryptococcal antigen—showed no atypical findings; tumour markers—including carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous cell carcinoma antigen—were also normal.
Chest CT showed a nodule measuring 10 mm located dorsally to the heart and descending aorta and obscured by bronchial shadowing, making visibility difficult by chest x-ray (figure).
A provisional diagnosis of lung metastasis from the rectal and anal cancer was made; the patient had a thoracoscopic partial pulmonary resection with no postoperative problems. Histopathological examination of a sample of tissue removed from the 12 mm × 8 mm lesion, during the operation, showed a moderate to well differentiated tubular adenocarcinoma confirming the diagnosis of lung metastasis from the rectal cancer.
At 3-month follow-up, no evidence of recurrence of cancer was seen on a repeat chest x-ray reviewed by a radiologist and the AI package. The patient reported that she was well with no respiratory symptoms.
In recent years, there have been many attempts to provide easy chest x-ray-based automated diagnosis using machine learning and deep learning. Computer-aided diagnosis is beginning to be incorporated in diagnostic processes, reducing physicians’ workloads.
The diagnostic performance of AI-based chest x-ray has reached the same accuracy as that of radiologists, and in some cases may exceed it.
(18). Amrou Sarraj et al. Endovascular thrombectomy plus medical care versus medical care alone for large ischaemic stroke: 1-year outcomes of the SELECT2 trial. The Lancet 403(10428): P731-740.
Summary
Background
Multiple randomised trials have shown efficacy and safety of endovascular thrombectomy in patients with large ischaemic stroke. The aim of this study was to evaluate long-term (ie, at 1 year) evidence of benefit of thrombectomy for these patients.
Methods
SELECT2 was a phase 3, open-label, international, randomised controlled trial with blinded endpoint assessment, conducted at 31 hospitals in the USA, Canada, Spain, Switzerland, Australia, and New Zealand. Patients aged 18–85 years with ischaemic stroke due to proximal occlusion of the internal carotid artery or of the first segment of the middle cerebral artery, showing large ischaemic core on non-contrast CT (Alberta Stroke Program Early Computed Tomographic Score of 3–5 [range 0–10, with lower values indicating larger infarctions]) or measuring 50 mL or more on CT perfusion and MRI, were randomly assigned, within 24 h of ischaemic stroke onset, to thrombectomy plus medical care or to medical care alone. The primary outcome for this analysis was the ordinal modified Rankin Scale (range 0–6, with higher scores indicating greater disability) at 1-year follow-up in an intention-to-treat population. The trial is registered at ClinicalTrials.gov (NCT03876457) and is completed.
Findings
The trial was terminated early for efficacy at the 90-day follow-up after 352 patients had been randomly assigned (178 to thrombectomy and 174 to medical care only) between Oct 11, 2019, and Sept 9, 2022. Thrombectomy significantly improved the 1-year modified Rankin Scale score distribution versus medical care alone (Wilcoxon-Mann-Whitney probability of superiority 0·59 [95% CI 0·53–0·64]; p=0·0019; generalised odds ratio 1·43 [95% CI 1·14–1·78]). At the 1-year follow-up, 77 (45%) of 170 patients receiving thrombectomy had died, compared with 83 (52%) of 159 patients receiving medical care only (1-year mortality relative risk 0·89 [95% CI 0·71–1·11]).
Interpretation
In patients with ischaemic stroke due to a proximal occlusion and large core, thrombectomy plus medical care provided a significant functional outcome benefit compared with medical care alone at 1-year follow-up.
Funding
Stryker Neurovascular.
(19). Lim, Emma & Parker, Emily & Vasey, Nicola. (2024). Why learning how to swallow pills is good for patients, parents, and the planet. BMJ. 384. e076257. 10.1136/bmj-2023-076257.
What you need to know
Pills are likely to have a reduced environmental impact compared with an equivalent dose of liquid medication, with less packaging and less wasted medicine
Pill swallowing is an important life skill that can improve dosing accuracy and adherence
Young patients often prefer pills to liquids. Pills contain fewer additives, need fewer doses, and have a longer shelf life
Children can successfully learn to swallow pills from the age of 4
Healthcare professionals and patients often assume that liquids are the most suitable oral medicinal formulation for children and young people. However, swapping liquids to pills can be safer, more cost effective, more acceptable to patients and carers, and is likely to reduce the carbon footprint of prescribing.
Why change is needed
Medicines and chemicals account for 25% of the NHS England carbon footprint.1 Few environmental impact studies compare liquid and tablet medicines, although available evidence suggests that pills have a lesser carbon footprint than equivalent liquid medications. A life cycle assessment based in India found that the carbon footprint of paracetamol pill production is 15 times less than an equivalent dose of liquid.2 Pills typically come in smaller, lighter packets than liquids, take up less space in distribution lorries, and create less packaging waste. Liquids also require dosing syringes or spoons, contributing to increased plastic waste.
Importantly, pills have a longer shelf life and can be stored out of the fridge so have lower energy requirements during their use, and are less likely to be discarded as a result of inadequate storage conditions. Pill packets can be divided up to dispense a specific number of doses, whereas liquid formulations must be dispensed in whole bottles, meaning excess doses from short term prescriptions (such as antibiotics)
(20). Herold KC, Gitelman SE, Gottlieb PA, Knecht LA, Raymond R, Ramos EL. Teplizumab: A Disease-Modifying Therapy for Type 1 Diabetes That Preserves β-Cell Function. Diabetes Care. 2023;46(10):1848-1856.
Treatment with teplizumab significantly preserved beta(ß)-cell function, as measured by C-peptide levels, in children and adolescents with new-onset type 1 diabetes (T1D) compared with placebo, in the PROTECT study
“In 2022, teplizumab, a humanized monoclonal antibody to CD3 on T cells, was approved by the FDA to delay the onset of clinical (stage 3) T1D in patients aged ≥8 years with preclinical (stage 2) T1D,” said the researchers.
“[However, it is unknown] whether treatment with intravenous teplizumab in patients with newly diagnosed T1D can prevent disease progression.

