Journal scan: A review of 26 recent papers of immediate clinical significance, harvested from major international journals
From the desk of the Editor-in-Chief
(1). Jonathan N. Hourmozdi et al, Myocardial Bridging February 1, 2024, N Engl J Med 2024; 390:e11.
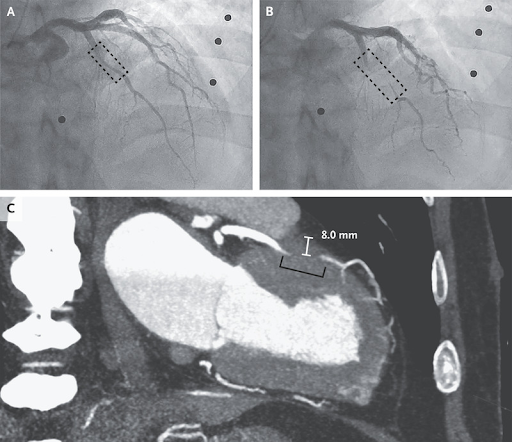
A 66-year-old man with a history of hypertension, diabetes mellitus, and ischemic stroke was transferred to a tertiary hospital after a cardiac arrest. For 6 months before presentation, he had had recurrent exertional angina but had not sought evaluation. On the morning of the cardiac arrest, he had woken with chest pain, lost consciousness, and regained consciousness after brief cardiopulmonary resuscitation by his family. On transfer to the tertiary hospital, findings from a physical examination and a transthoracic echocardiogram were normal. Coronary angiography revealed 50% stenosis in the middle left anterior descending (LAD) coronary artery during diastole (Panel A, dashed box) with complete occlusion during systole (Panel B, dashed box) and sluggish distal flow (see video). A diagnosis of myocardial bridging was made. Myocardial bridging is a coronary anomaly in which an epicardial coronary artery takes an intramuscular course. The condition is congenital but may not result in symptoms until later in life when concurrent left ventricular hypertrophy, coronary microvascular disease, or intraluminal stenosis develops from atherosclerosis. Coronary computed tomographic angiography that was performed for surgical planning showed an 8-mm depth of the middle LAD coronary artery in the myocardium (Panel C, bracket). Coronary-artery bypass surgery was performed with a saphenous vein graft. The patient recovered well and had no recurrence of symptoms.
(2). Weisheng Yang et al, February 1, 2024, , Incarcerated Obturator Hernia
Engl J Med 2024; 390:455, DOI: 10.1056/NEJMicm2309235
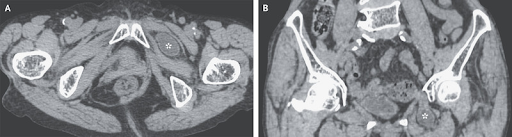
An 84-year-old woman with a history of six vaginal deliveries presented to the gastroenterology department with a 12-hour history of left lower abdominal pain, nausea, and vomiting. Her body-mass index (the weight in kilograms divided by the square of the height in meters) was 16, indicating underweight status. On physical examination, the patient had tenderness to palpation in the left inguinal region. Computed tomography of the abdomen revealed a loop of small bowel protruding through the left obturator canal, between the pectineus muscle anteriorly and the obturator external muscle posteriorly (Panels A [axial view] and B [coronal view], asterisk). Small-bowel dilatation with fluid accumulation proximal to the herniated bowel was also observed. A diagnosis of an incarcerated obturator hernia was made. Emergency laparotomy was performed, during which a part of the ileum located 80 cm from the ileocecal region was found embedded in the left obturator canal. The ileum was manually returned to the peritoneum, and the hernia was repaired. An obturator hernia is a rare type of hernia most commonly identified in thin, multiparous, older women. Owing to the lack of overt findings associated with this pelvic hernia on physical examination, diagnosis may be delayed. The patient recovered well and was discharged home 7 days after surgery.
(3). Hajar Dauleh, et al, January 25, 2024. Adjuvant Alpelisib Therapy for Congenital Hyperinsulinism. N Engl J Med 2024; 390:379-380,DOI: 10.1056/NEJMc2312807
Congenital hyperinsulinism is a cause of severe hypoglycemia in newborns. Mutations in ABCC8 and KCNJ11, genes that encode SUR1 and Kir6, respectively, cause medically unresponsive congenital hyperinsulinism, a condition that requires a near total pancreatectomy, leading to lifelong diabetes mellitus and pancreatic exocrine insufficiency.1 Thus, new treatment options are being sought for managing congenital hyperinsulinism.
Alpelisib is an -selective phosphatidylinositol 3-kinase (PI3K) inhibitor that was approved for the treatment of PIK3CA-mutated breast cancer and overgrowth syndromes.2,3 PI3K is a component of a cascade that regulates cell growth, proliferation, and development and insulin signaling. Inhibition of the PI3K pathway leads to decreased glucose transport and increased glycogenolysis and gluconeogenesis. Hyperglycemia (with diabetic ketoacidosis) is the most common adverse effect of alpelisib therapy.4 In a previous case report, treatment with alpelisib in a patient with intractable non islet-cell tumor hypoglycemia led to an improvement in the glucose profile.5 Children with hypoglycemia due to mutations in PIK3CA who are treated with alpelisib do not have any growth-related problems.3
We report a case of severe congenital hyperinsulinism due to homozygous deletion of the ABCC8 gene in a patient who had had no response to conventional medical therapies (i.e., diazoxide, nifedipine, hydrocortisone, and octreotide). At the referring hospital, the patient had begun receiving a combination of intravenous dextrose therapy, continuous gastrostomy-tube feeding, and injections of octreotide LAR (long-acting release; 15 mg once every 4 weeks) to maintain acceptable blood glucose levels.
Treatment Progress with Adjuvant Alpelisib Therapy over a 13-Week Period.
The patient was referred to our center at 4 months of age. After obtaining approval from the pharmaceutical and therapeutics committee, we repurposed alpelisib for the treatment of congenital hyperinsulinism and started with the minimum dose of 12.5 mg once daily. The dose was gradually increased to the maximum tolerated dose of 30 mg twice daily, and 6 weeks after starting treatment, intravenous dextrose therapy was discontinued and continuous gastrostomy-tube feeding was changed to bolus feeding every 3 hours (Figure 1). In the background, the injections of octreotide LAR once every 4 weeks were continued. Continuous glucose monitoring showed a substantial reduction in the percentage of time below the target range (63 mg per deciliter [3.5 mmol per liter]) over time, with a value of only 2% at 13 weeks. The hyperglycemic effect of alpelisib can vary from 4 hours to 4 weeks among adults.4 The only adverse effects that were observed were vomiting, retching, and a mild elevation in the serum insulin level, and these effects were dose-dependent (>30 mg twice daily). The patient’s weight and length have remained at the 50th percentile for age before and after treatment with alpelisib.
These observations suggest that alpelisib may be used as an adjuvant therapy for patients with congenital hyperinsulinism that is otherwise medically unresponsive and that this therapy may help in avoiding a near total pancreatectomy. Further clinical trials are warranted to assess the efficacy and safety of alpelisib in patients with congenital hyperinsulinism.
(4). Karl Njuwa Fai et al, January 11, 2024, N Engl J Med 2024; 390:166-173,DOI: 10.1056/NEJMcpc2301033
A 25-year-old pregnant woman presented to the emergency department of the Poli District Hospital in the North Region of Cameroon because of vaginal bleeding after a snakebite.
The patient had been in her usual state of health until 1 hour before this presentation, when she was bitten by a snake while walking with her family.
On presentation to the emergency department, she reported fatigue, dizziness, abdominal pain, and pain and numbness in her left lower leg. The Glasgow Coma Scale score was 15 (on a scale of 3 to 15, with lower scores indicating greater alteration of consciousness).
On examination, the heart rate was 134 beats per minute, the blood pressure 100/60 mm Hg, and the respiratory rate 20 breaths per minute while the patient was breathing ambient air. The physical examination was notable for mild conjunctival pallor and swelling in the inferior left lower leg, which had been tied with a tourniquet.
The leg was tender with ecchymosis, and two fang marks were visible on the left foot (Figure 1). The patient had diffuse pain in the lower abdomen, intermittent contractions, and vaginal bleeding. Fetal movement was detected on palpation. The fetal heart rate was 112 beats per minute; ultrasonography was not available. The remainder of the physical examination was normal.
The hemoglobin level was 7.0 g per deciliter (reference range, 11.5 to 15.0), the urea nitrogen level 145 mg per deciliter (51.8 mmol per liter; reference range, 15 to 45 mg per deciliter [5.4 to 16.1 mmol per liter]), the creatinine level 8.9 mg per deciliter (786.8 mol per liter; reference range, 0.6 to 1.3 mg per deciliter [53.0 to 114.9 mol per liter]), and the platelet count 36,000 per microliter (reference range, 150,000 to 450,000). Urine dipstick testing was positive for blood, and a 20-minute whole-blood clotting test revealed clotting abnormalities (Figure 2).
The patient lived with her family in the North Region of Cameroon. She reported that she was at approximately 7 months’ gestation and had received no prenatal care. She had a history of six previous pregnancies. There was no other relevant medical or family history. She had been accompanied to the emergency department by her family members, who had brought the dead snake with them for identification .Management decisions were made.
Differential Diagnosis
Dr. Karl Njuwa Fai: I am aware of the final diagnosis in this case. This 25-year-old pregnant woman presented to the hospital with vaginal bleeding, lower abdominal pain, and a viable fetus. Other relevant clinical features included a low platelet count, possible hematuria, elevated blood levels of urea nitrogen and creatinine, a low hemoglobin level, and clotting abnormalities, as measured by the 20-minute whole-blood clotting test. In a resource-limited setting such as this one, the diagnosis is typically made on the basis of the clinical presentation and medical history of the patient. The differential diagnosis for bleeding in the third trimester of pregnancy can be grouped into causes that are related to pregnancy and those that are not related to pregnancy.
Pregnancy-Related Causes of Bleeding
Pregnancy-related vaginal bleeding in the third trimester of pregnancy is commonly caused by placenta previa, placental abruption, ruptured uterus, or subchorionic hemorrhage.
Placenta Previa
Placenta previa, a condition in which all or part of the placenta is implanted into the lower segment of the uterus, is the most likely cause of bleeding in the third trimester of pregnancy. This patient presented to the hospital at an estimated 7 months’ gestation with vaginal bleeding and intermittent contractions, findings that are consistent with a diagnosis of placenta previa. She also had a history of multiparity at a young age, which is a risk factor for placenta previa. Other risk factors, which were not present in this patient, are a history of abortion, a history of placenta previa, multiple gestation, fibroids, advanced maternal age, and the use of tobacco products. Placenta previa can be diagnosed by means of ultrasonography during the second trimester of pregnancy. However, since this patient had not received any prenatal care, she had not undergone an ultrasound examination. Also, patients who present with placenta previa are unlikely to have clotting abnormalities detected at the time of presentation, whereas this patient had a low platelet count and coagulopathy, as measured by the 20-minute whole-blood clotting test. Although the resources were not available to perform an ultrasound examination, a vaginal examination was performed to rule out the presence of a low-lying placenta.
Placental Abruption
Placental abruption is early, extensive detachment of the placenta during pregnancy or premature detachment of a normally implanted placenta that causes blood to accumulate between the placenta and the uterus. Although this patient presented with vaginal bleeding, she did not have abdominal pain that was sudden in onset or constant or stabbing in nature, and such pain would be characteristic of placental abruption. Furthermore, the vaginal blood was not described as dark, and the patient did not present with signs of preeclampsia. Placental abruption can be associated with a history of arterial hypertension, preeclampsia, abdominal trauma, folic acid deficiency, or the use of tobacco products or cocaine, none of which were reported by the patient. The diagnosis of placental abruption is usually made by means of ultrasound examination.
Uterine Rupture
Although uterine rupture is an unlikely diagnosis in this patient owing to the absence of risk factors such as a scarred uterus, direct trauma, and mechanical dystocia, it is important to consider as a cause of bleeding in the third trimester of pregnancy since it has clinically significant implications for both the mother and the fetus. Patients with uterine rupture often present with acute, intense pain with minimal vaginal bleeding. On physical examination, the fetus is high-riding in the uterus, and the fetal parts can be detected on palpation; in this case, the fetus was low-lying in the uterus.
Subchorionic Hemorrhage
Subchorionic hemorrhage is a less common cause of bleeding in the third trimester of pregnancy than placental abruption or uterine rupture. This patient presented with bright red vaginal blood, not dark red, and she had tachypnea and tachycardia, findings that are not consistent with a diagnosis of subchorionic hemorrhage.
Causes of Bleeding Not Related to Pregnancy
Although it is always important to consider pregnancy-related causes of vaginal bleeding in an otherwise healthy pregnant patient, the low platelet count and the development of coagulopathy are the most likely explanations for this patient’s bleeding. Given that she reported that a snakebite had occurred approximately 1 hour before presentation, envenomation is the most likely diagnosis for her bleeding disorder. The physical examination confirmed the presence of two fang marks on her left foot. Venom from certain types of snakes can cause coagulopathy and trigger the development of intermittent contractions in pregnancy due to the effects of snake venom phospholipase A2 (PLA2). Standardized assays of fibrinogen (and fibrin degradation products) and prothrombin time are the most sensitive assays for the diagnosis of coagulopathy induced by snake venom; however, these tests were not available in this hospital.
In the North Region of Cameroon, this type of coagulopathy can be caused by the venom from a carpet viper bite. On the basis of its classic appearance, the dead snake was identified as Echis ocellatus, also known as the carpet viper, which established the diagnosis of disseminated intravascular coagulation due to carpet viper envenomation.
Dr. Karl Njuwa Fai’s Diagnosis:
Disseminated intravascular coagulation due to envenomation by a carpet viper (Echis ocellatus).
Discussion of Pathophysiology
Dr. Fai: The most venomous snake in sub-Saharan Africa, E. ocellatus, has venom composed of complex enzymes, peptides, and metalloproteinases.1 Snake venom triggers a robust inflammatory response with the synthesis and release of several substances, including nitric oxide, complement anaphylatoxins, histamine, cytokines, and eicosanoids. Macrophages and leukocytes are mobilized to the affected area, which leads to vascular permeability and the formation of an exudate. Exudates contribute to inflammation and tissue damage.
Viper venom contains phospholipases (e.g., PLA2), metalloproteinases, serine proteinases, and other cytotoxins that mediate most of the clinical manifestations after a bite occurs. The PLA2 enzymes cause myonecrosis by breaking down membrane phospholipids of muscle fibers or through hydrophobic interactions. This disruption of the plasma membrane is associated with an influx of calcium into the muscle cells, leading to the sustained contraction of myofilaments, irreversible damage of myocytes, and dysfunction of mitochondria. This muscle damage is aggravated by the action of snake venom metalloproteinases that break down type IV collagen, an essential component of the capillary basement membrane. Snake venom metalloproteinases and hyaluronidases induce microvascular damage and affect the function of other tissues.
Snake venom metalloproteinases and serine proteinases are procoagulant proteins that can not only activate coagulation factors such as factor V, factor X, and prothrombin but also act as thrombinlike enzymes. In addition, they can hydrolyze fibrinogen and fibrin. The expression of C-type lectin proteins and the microvascular damage caused by snake venom metalloproteinases can result in a decreased platelet population by blocking platelet receptors or directly interacting with von Willebrand factor. These processes all lead to disseminated intravascular coagulation and may contribute to systemic bleeding.
Pathophysiological Features of Envenomation by a Carpet Viper.
The direct cytotoxic action of PLA2s, coupled with the ensuing ischemia due to systemic bleeding, vascular damage, and degradation of the glomerular basement membrane by snake venom metalloproteinases, cause acute kidney injury. In addition, the effects of PLA2s on the membrane phospholipids and adenylate cyclase activity of smooth muscle of the uterus could have led to labor in this patient..
Discussion of Management
Dr. Linda Esso: The management of snakebites varies widely, depending on the identification of the snake involved. Not all snakes have venom, so not all patients need antivenom. It is important to remember that antivenom may still be efficacious even if it is administered days after the bite. Therefore, in situations in which the identity of the snake is unknown, it may be prudent to wait for the development of toxic effects rather than administering antivenom empirically.2-5
The management of snake envenomation involves several important steps. First aid consists of reassuring the patient and keeping that person immobile, which can help slow the spread of the venom in the body. When possible, the patient should be placed in the recovery position (on the side with the upper leg bent to protect the airway and aid in drainage in case of vomiting).
A rapid clinical assessment is performed, along with resuscitation if necessary. Then, a more detailed assessment is done, and the snake is identified if possible. Laboratory testing is performed, with an emphasis on coagulation tests. The 20-minute whole-blood clotting test is a simple test of coagulability that is available in most resource-limited settings, and it can be used at the bedside for the detection of coagulopathy. Analgesic agents are usually administered for pain.
Treatment with antivenom may be indicated when the patient has one or more signs of envenomation, such as hemostatic abnormalities, neurotoxic signs, local swelling involving more than half the affected limb or rapid extension of swelling, the development of an enlarged lymph node draining the affected limb, cardiovascular abnormalities, acute kidney injury, or supporting laboratory evidence of systemic envenomation. The administration of antivenom can be crucial in the treatment of envenomation; however, the decision to use antivenom ultimately depends on the patient’s clinical status, the species of the snake, and the venom’s potential toxic effects. Antivenom would be used if the expected benefits exceed the calculated risks. Most antivenoms are administered intravenously with close monitoring of the patient so that adverse reactions related to the antivenom can be quickly identified and treated. Epinephrine is available during the administration of antivenom to limit any severe adverse reactions, including acute toxic effects (e.g., anaphylaxis or fever) and delayed reactions (e.g., serum sickness). The acute reactions are usually mild, but severe systemic anaphylaxis may occur, often within approximately 1 hour after exposure to antivenom.
If the patient is in shock, intravenous fluid is administered for resuscitation. The bite wound is cleaned and examined for signs of infection or tissue necrosis and for evidence of incisions made by family members or traditional healers to bleed the venom before the patient’s arrival at the hospital. Tetanus toxoid and antibacterial therapy are typically administered to patients who are in shock. Once immediate care is provided, the patient is usually assessed for transfer to a specialized health care center.
Follow-up
Dr. Moustafa: We administered two vials of antivenom (Inoserp PAN-AFRICA) diluted in 250 ml of normal saline immediately on the patient’s arrival in the emergency department. Second and third doses were administered 3 and 6 hours, respectively, after the first dose. Despite the administration of antivenom, a 20-minute whole-blood clotting test showed no clot formation.
On the second hospital day, the hemoglobin level remained at 7 g per deciliter. Two pints of fresh whole blood were transfused. Analgesics, glucocorticoids, and promethazine were also administered. The rest of the day was uneventful.
On the third hospital day, worsening vaginal bleeding and pelvic pain developed in the patient, features consistent with labor. A fourth dose of antivenom was administered. On a physical examination performed 3 hours later, the cervix was 5 cm dilated, and there was no bleeding. The pelvic pain persisted, and a 20-minute whole-blood clotting test showed findings consistent with persistent coagulopathy.
On the fourth hospital day, the patient delivered a live baby boy. The infant’s weight was 3400 g, a weight indicative of a near-term or full-term gestation. A moderate cervical tear and bleeding had occurred during labor, but the bleeding ceased with repair of the cervix. An additional 4 pints of fresh whole blood were transfused. Approximately 2 hours later, the vaginal bleeding recurred; another dose of antivenom was administered, and an additional 2 pints of fresh whole blood were transfused. The patient was transferred by automobile to the Garoua Regional Hospital, a tertiary care hospital located approximately 3 to 4 hours from Poli.
On arrival at the second hospital, the patient had stopped bleeding, but a diagnosis of acute kidney failure was made on the basis of a serum creatinine level of 8.9 mg per deciliter. An additional 2 pints of fresh whole blood were transfused. Despite aggressive treatment with antivenom and blood transfusion, 4 days after a snakebite by a carpet viper, the patient died.
Advocacy and Call to Action
Dr. Yap Boum II: Snakebites are a public health problem. Each year, snakebites occur in approximately 5 million people worldwide, causing about 125,000 deaths and 400,000 disabilities annually.7 In tropical climates such as sub-Saharan Africa, the burden is substantial, with about 20,000 deaths annually.
In 2015, a surveillance system was created in Cameroon to monitor mortality, enhance early detection and response, and produce evidence of the burden of snakebite disease in the country. Between 2018 and 2022, Cameroon registered 40 snakebite cases associated with 1056 deaths (2.6%). The Far North, Center, and North Regions are the most affected areas of the country, with more than 1500 snakebite cases reported per year.
Snakebites Occurring in Pregnancy
Snakebites in pregnant patients are a rare occurrence, but when they do occur, they are associated with substantial morbidity and mortality for both the mother and the fetus.9,10 Snakebites occurring in pregnancy are not often documented in the literature. Studies conducted in South Africa, Sri Lanka, and India showed that the prevalence of snakebites occurring in pregnancy ranged from 0.4 to 1.8%. Although rare, they can be associated with very severe adverse outcomes, as was seen in this patient, with maternal death occurring in up to 4% of reported cases and fetal loss in 20%.10 Diverse outcomes are possible for the mother and the fetus, depending on several factors, including the type of snake and the gestational age of the fetus. Fetal complications can include deformation, prematurity, and in severe cases, death. As seen in this case, maternal complications can include antepartum hemorrhage, coagulopathy, spontaneous abortion, labor, and kidney injury.1Antivenoms can be used to treat snakebites in pregnant patients even though their safety has not been established in this population; however, they are not widely accessible because of their cost and a lack of availability.
Tmely medical treatment and the early administration of antivenom, when indicated, have been shown to influence the outcomes of snakebites in pregnancy. This patient arrived at the hospital for medical attention approximately 1 hour after the snakebite had occurred, which is earlier than most other patients in this region would arrive. Patients typically arrive 2 to 3 days after the snakebite, after they have sought care from traditional healers and received treatments associated with limited scientific evidence of improved outcomes. The availability and affordability of antivenom is a notable issue in Africa. It is usually difficult to obtain antivenom in the remote areas of Cameroon, where the highest number of snakebites occurs. When antivenom is available, the cost is high ($100 to $200 per dose) for these communities, which have an average monthly income of less than $60 per person. This patient had immediate access to antivenom because the hospital was participating in a pharmacovigilance study that provided antivenom for free. Changes in the attitudes of international health stakeholders (e.g., community leaders, pharmaceutical companies, the Ministry of Public Health, Africa Centers for Disease Control and Prevention, and the World Health Organization) toward snakebites, universal health coverage, and local production of antivenom can go a long way in making antivenom more affordable.
The observed complications in this patient were coagulopathy, obstetric hemorrhage, cervical tear, hypovolemic shock, anemia, and labor and delivery. The baby survived, but the mother died. A combination of the systemic envenomation from a carpet viper bite and the resulting complications led to the fatal outcome in the mother. Immediate delivery of the fetus certainly played an important part in the survival of the baby.
The personnel of Poli District Hospital had been trained in the rapid bedside diagnosis of coagulopathy with the use of the 20-minute whole-blood clotting test in the absence of hematologists and well-equipped laboratories. Although the health care providers made an early diagnosis of anemia and coagulopathy, the hospital had an insufficient supply of appropriate blood products such as clotting factors. In this case, the patient received a total of 10 pints of fresh whole blood.
Snakebites pose a substantial threat to the health and well-being of communities across Africa. Venomous bites often lead to serious complications, disability, and even death if not treated promptly and effectively. The management of this neglected tropical disease depends on rapid detection and the administration of adequate antivenom. Antivenom is often the most effective treatment for snakebites since it neutralizes the venom, preventing further damage. In Cameroon, only the Inoserp antivenom, which is not effective against all snake venoms, is available. Because of its cost, this antivenom is not available at all health care facilities, and it is not affordable for the at-risk populations in the tropics, including rural communities and indigenous populations that practice farming. Promoting local production and advocating for the availability and accessibility of snakebite antivenoms in Africa is therefore of paramount importance. Improved access to affordable antivenom, snakebite prevention, and community education can save lives, reduce the burden on health systems, and help achieve the Sustainable Development Goals. Together, we can make a substantial difference in the fight against snakebite and improve the health of African people.
Final Diagnosis
Carpet viper (Echis ocellatus) envenomation.
(5). John L. Sapp et al, RAFT Long-Term Study Team, January 18, 2024. Long-Term Outcomes of Resynchronization Defibrillation for Heart Failure. N Engl J Med 2024; 390:212-220.
Abstract
Background
The Resynchronization Defibrillation for Ambulatory Heart Failure Trial (RAFT) showed a greater benefit with respect to mortality at 5 years among patients who received cardiac-resynchronization therapy (CRT) than among those who received implantable cardioverter defibrillators (ICDs). However, the effect of CRT on long-term survival is not known.
Methods
We randomly assigned patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of 120 msec or more (or a paced QRS duration of 200 msec or more) to receive either an ICD alone or a CRT defibrillator (CRT-D). We assessed long-term outcomes among patients at the eight highest-enrolling participating sites. The primary outcome was death from any cause; the secondary outcome was a composite of death from any cause, heart transplantation, or implantation of a ventricular assist device.
Results
The trial enrolled 1798 patients, of whom 1050 were included in the long-term survival trial; the median duration of follow-up for the 1050 patients was 7.7 years (interquartile range, 3.9 to 12.8), and the median duration of follow-up for those who survived was 13.9 years (interquartile range, 12.8 to 15.7). Death occurred in 405 of 530 patients (76.4%) assigned to the ICD group and in 370 of 520 patients (71.2%) assigned to the CRT-D group. The time until death appeared to be longer for those assigned to receive a CRT-D than for those assigned to receive an ICD (acceleration factor, 0.80; 95% confidence interval, 0.69 to 0.92; P=0.002). A secondary-outcome event occurred in 412 patients (77.7%) in the ICD group and in 392 (75.4%) in the CRT-D group.
Conclusions
Among patients with a reduced ejection fraction, a widened QRS complex, and NYHA class II or III heart failure, the survival benefit associated with receipt of a CRT-D as compared with ICD appeared to be sustained during a median of nearly 14 years of follow-up.
(6). Yan Gao et al, Challenges in Clinical Electrocardiography Recurrent Ventricular Fibrillation in an Apparently Healthy Patient. JAMA Intern Med. 2024;184(2):205-206. doi:10.1001/jamainternmed.2023.5244
Case Presentation
Awoman in her 50s was brought to the emergency department after she collapsed, received chest compressions from her husband, and recovered consciousness at home. On admission, the patient was conscious without any discomfort. Vital signs were normal and physical examination was unremarkable. A 12-lead electrocardiogram (ECG) on admission was recorded (Figure, A). Shortly after admission, continuous ECG monitoring (Figure, B) revealed recurrent episodes of polymorphic ventricular tachycardia (VT). Although most episodes did not sustain, some deteriorated into ventricular fibrillation (VF) associated with loss of consciousness and required direct current cardioversion. Intravenous infusions of lidocaine, amiodarone, magnesium, and isoproterenol failed to prevent recurrence of these malignant arrhythmias. Serum electrolyte levels were normal. Transthoracic echocardiography showed normal cardiac function and structure. Emergency coronary angiography showed no evidence of obstructive coronary artery disease. A temporary transvenous right ventricular pacemaker was inserted. However, ventricular overdrive pacing was not effective in suppressing the polymorphic VT. Her condition deteriorated and, over the next hour, she required direct current cardioversion 12 times.
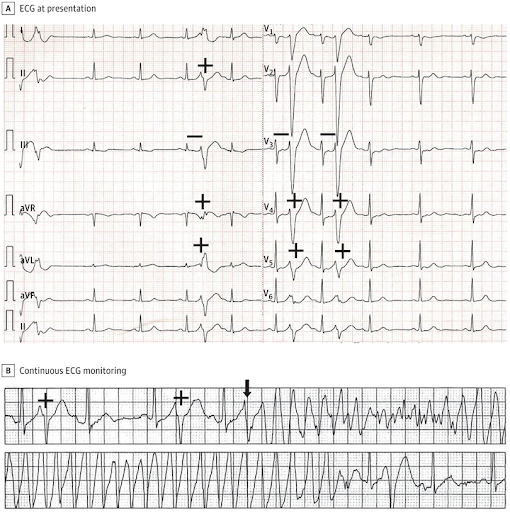
A, The patient’s 12-lead ECG showed remarkable for repetitive monomorphic premature ventricular complexes (+) that had an atypical left bundle-branch block/left axis deviation like morphology and a short-coupling interval of 240 milliseconds (−). B, the patient’s rhythm strip showed a sinus rhythm with frequent premature ventricular depolarizations (+). One of these premature ventricular depolarizations (arrow) induced polymorphic ventricular tachycardia that may degenerate into ventricular fibrillation.
Questions: What is the electrocardiographic diagnosis for this patient, and what is the best course of treatment?
Interpretation
The patient’s 12-lead ECG (Figure, A), and particularly the rhythm strip at the onset of polymorphic VT/VF (Figure, B) showed sinus rhythm with normal QRS and QT intervals, normal TU waves, no J-point or ST-segment elevation, and no conduction abnormalities. The patient’s 12-lead ECG was remarkable for repetitive monomorphic premature ventricular complexes that had an atypical left bundle-branch block/left axis deviation like morphology and a short-coupling interval of 240 milliseconds (Figure, A) that may induce polymorphic VT that may degenerate into VF (Figure, B). These features are typical of the short-coupled polymorphic VT/VF (SC-PMVT/VF) variant of idiopathic VF.
After an intravenous bolus (10 mg) and a drip of verapamil (5 mg per hour) was administered, the ventricular arrhythmias were controlled within 15 minutes. During the following 3 days of hospitalization, the patient received a continuous intravenous drip of verapamil and remained arrhythmia free. The patient declined to receive either radiofrequency catheter ablation and an implanted cardioverter-defibrillator (ICD). After discharge, the patient took oral verapamil, 240 mg, per day. During 6 months of follow-up, the patient did not have any further episodes of syncope. The 24-hour ambulatory ECG showed that the patient was no longer having short-coupled premature ventricular complexes. Genetic testing suggested a variant of unknown significance in the coding gene of cardiac ryanodine receptor (RyR2).
Discussion
The patient has no evident heart disease, and there was no evidence of a subclinical ventricular arrhythmia syndrome (such as long QT syndrome, short QT syndrome, early repolarization syndrome, arrhythmogenic right ventricular dysplasia, or Brugada syndrome). This patient’s VF arrest was appropriately classified as idiopathic VF. The term SC-PMVT/VF refers to a patient resuscitated from a cardiac arrest, preferably with documented VF that has been clinically evaluated to exclude known cardiac, metabolic, respiratory, and toxicological causes.1 SC-PMVT/VF is a specific idiopathic VF subtype. Typically, monomorphic premature ventricular complexes (most often but not always) with an atypical left bundle-branch block/left axis deviation like QRS morphology and a very short (350 milliseconds) coupling interval induces polymorphic VT that may degenerate into VF.2
The entity of SC-PMVT/VF has been recognized since the 1990s. However, early reports likely described heterogeneous study populations that included undiagnosed Brugada syndrome, short QT interval syndrome, and early repolarization syndrome in addition to SC-PMVT/VF.3 In 1994, Leenhardt et al4 observed that idiopathic VF induced by short-coupled premature ventricular complex had the characteristic twisting of the points appearance of torsade, and first used the term short-coupled torsades de pointes. Nevertheless, the use of the term torsades de pointes in this setting is confusing as torsade de pointes has come to be tightly associated with the long QT interval syndromes. Given that the short-coupled ventricular premature complex induces polymorphic VT, which may or may not degenerate into VF, the term SC-PMVT/VF is preferred to the term short-coupled VF.
SC-PMVT/VF is malignant with recurrent arrhythmias. SC-PMVT/VF is responsible for about 3% to 5% of VT/VF cardiac arrests; nevertheless, very few practitioners are aware that the entity and its unusual treatment exist.5 The usual medical treatments for electrical storm, including β-blockers and amiodarone, are frequently ineffective. Although intravenous injection then oral quinidine therapy appears to be the most effective therapy (perhaps by blocking the Ito current), these therapies are usually not immediately available because very few treatment centers have immediate access to oral quinidine and even fewer have immediate access to intravenous quinidine. Accordingly, consideration of the diagnosis permits use of the second most effective antiarrhythmic drug therapy, that of verapamil. Calcium channel blocking agents, especially verapamil, are often effective treatment in this setting by prolonging the premature ventricular complex coupling interval and by suppressing the premature ventricular complexes.4 As the initiating premature ventricular complexes are focal in origin, patients with SC-PMVT/VF are also suitable for transcatheter ablation. In more than 80% of patients with SC-PMVT/VF, the 5-year recurrence of VF or polymorphic VT can be prevented.6 However, neither verapamil nor radiofrequency can completely eliminate the risk of recurrent VF or VT. Therefore, ICD placement is still recommended for most patients with SC-PMVT/VF.7
Take-Home Points
The diagnosis of SC-PMVT/VF should be considered in patients without evident underlying heart disease whose polymorphic VT/VF is initiated by a premature ventricular complex with a short coupling interval (350 milliseconds).
Calcium channel blocking agents, especially verapamil, are often effective treatment for SC-PMVT/VF by prolonging the premature ventricular complex coupling interval and by suppressing the premature ventricular complexes.
Practitioners should be aware of SC-PMVT/VF and its appropriate treatment, including appropriate management with calcium channel blockers, ablation, and ICD placement.
(7). Hirotaka Yamamoto et al, February 8, 2024, Interstitial Cystitis in Sjgren’s Syndrome. N Engl J Med 2024; 390:548,DOI: 10.1056/NEJMicm2308925
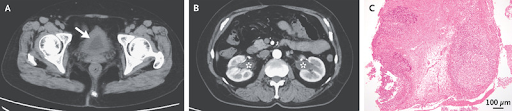
A 66-year-old woman with primary Sjgren’s syndrome presented to the rheumatology clinic with a 1-month history of lower abdominal pain and urinary urgency. One year before this presentation, she had received a diagnosis of Sjgren’s syndrome after presenting with sicca symptoms and arthralgias. Subsequent oral administration of pilocarpine had controlled her symptoms. The physical examination was notable for suprapubic pain and proximal interphalangeal joint tenderness. Laboratory testing showed elevated inflammatory markers, mild acute kidney injury, and mild hematuria and pyuria on urinalysis. A urine culture was negative. Computed tomography of the abdomen showed bladder-wall thickening (Panel A, arrow) and hydronephrosis in both kidneys without nephrolithiasis (Panel B, asterisks). The results of bladder biopsy were negative for cancer. Histopathological analysis showed lymphocytic and plasmacytic infiltration, ulceration, and fibrin deposition of the bladder wall, as well as lymphoid follicle formation (Panel C, hematoxylin and eosin stain). A diagnosis of interstitial cystitis associated with Sjgren’s syndrome a rare extraglandular feature of the autoimmune condition was made. Oral prednisolone and mycophenolate mofetil were initiated. The patient’s renal function improved after 3 months of treatment, and her urinary symptoms had abated by 4 months. Repeat imaging that was performed 6 months after the initial presentation showed resolution of the hydronephrosis
(8). Jrme Antonelli etal,. Transient Monocular Blindness in a Man in His 30s
JAMA Cardiol.Published online February 7, 2024.
A patient in his 30s presented to the emergency department with transient monocular blindness but no cardiovascular symptoms. Cerebral magnetic resonance imaging was normal, and a transient ischemic attack (TIA) was diagnosed. Transthoracic echocardiography revealed a gigantic, dilated structure (Figure, A) corresponding to the ascending aorta, with severe stenotic type 0 bicuspid aortic valve. Computed tomography confirmed a massive, 122-mm ascending aortic dilation (3-dimensional reconstruction inFigure, B) with signs of prerupture, confined between the sternum and the spinal cord, compressing the left atrium. An urgent bioprosthetic Bentall surgery was performed.
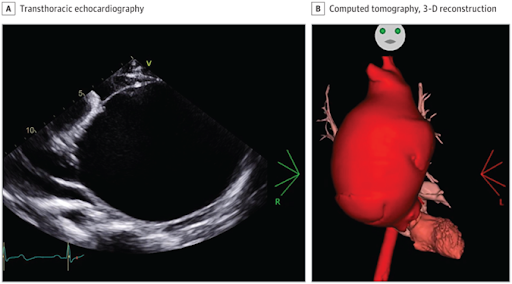
Giant ascending aortic aneurysms, defined as exceeding 10 cm in diameter, are rare entities, especially in recent years due to the widespread use of echocardiography.1 Aortic dilatation may be asymptomatic for years and manifest at the time of a complication, such as a TIA. Transthoracic echocardiography should always be performed to rule out cardiac origin.
(9). Lievens Y. Modelling radiotherapy availability in the Asia-Pacific region. Lancet Oncol. 2024 Feb;25(2):152-154.
Abstract
Radiotherapy is a cornerstone in the multidisciplinary approach to cancer care. In 2012, the Global Task Force on Radiotherapy for Cancer Control estimated that, if all patients with cancer with an evidence-based indication for radiotherapy worldwide had access to radiotherapy, more than 580 000 patients would be cured each year, amounting to 1 million by 2035.
These figures pertain to the global cancer population, including patients that receive palliative radiotherapy, for whom long-term benefits are not anticipated, and even those for whom radiotherapy is not indicated
(10). Jakob Christensen et al. Prenatal exposure to antiseizure medications and fetal growth: a population-based cohort study from the Nordic countries.https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(24)00015-2/fulltext
Summary
Background
The short- and long-term consequences of restricted fetal growth cause considerable concern, and how prenatal exposure to different antiseizure medications (ASMs) affects fetal growth remains uncertain.
Methods
This was a population-based cohort study of liveborn singleton children born in Denmark, Finland, Iceland, Norway, and Sweden from 1996 to 2017. Prenatal exposure was defined as maternal filling of prescriptions for ASM during pregnancy registered in national prescription registries and primary outcomes were adjusted odds ratios (aORs) of microcephaly or being born small for gestational age.
Findings
We identified 4,494,918 children (males: 51.3%, 2,306,991/4,494,918), including 38,714 (0.9%) children of mothers with epilepsy. In the overall population, prenatal monotherapy exposure with carbamazepine (aOR: 1.25 (95% CI: 1.12 1.40)), pregabalin (aOR: 1.16 (95% CI: 1.02 1.31)), oxcarbazepine (aOR: 1.48 (95% CI: 1.28 1.71)), clonazepam (aOR: 1.27 (95% CI: 1.10 1.48)), and topiramate (aOR: 1.48 (95% CI: 1.18 1.85)) was associated with risk of being born small for gestational age, and carbamazepine was associated with microcephaly (aOR: 1.43 (95% CI: 1.17 1.75)). In children of mothers with epilepsy, prenatal exposure to carbamazepine (aOR: 1.27 (95% CI: 1.11 1.47)), oxcarbazepine (aOR: 1.42 (95% CI: 1.18 1.70)), clonazepam (aOR: 1.40 (95% CI: 1.03 1.89)), and topiramate (aOR: 1.86 (95% CI: 1.36 2.54)) was associated with being born small for gestational age; carbamazepine, with microcephaly (aOR: 1.51 (95% CI: 1.17 1.95)). No associations with small for gestational age and microcephaly were identified after prenatal exposure to lamotrigine, valproate, gabapentin, levetiracetam, phenobarbital, acetazolamide, phenytoin, clobazam, primidone, zonisamide, vigabatrin, ethosuximide and lacosamide, but except for lamotrigine, valproate, gabapentin, and levetiracetam, numbers of exposed children were small.
Interpretation
Prenatal exposure to carbamazepine, oxcarbazepine, clonazepam, and topiramate was associated with increased risk of being born small for gestational age in both the overall population and in children of women with epilepsy suggesting that prenatal exposure to these drugs is associated with fetal growth restriction.
Funding
The NordForsk Nordic Program on Health and Welfare (83539), the Independent Research Fund Denmark (1133-00026B), the Danish Epilepsy Association, the Central Denmark Region, the Novo Nordisk Foundation )
(11). María Paula Abaunza-Camach et al. Localised re-expansion pulmonary oedema following spontaneous pneumothorax.
A 53-year-old man presented to our emergency department with a 2-week history of increasing dyspnoea and a dry cough.
The patient had a history of chronic obstructive pulmonary disease recently diagnosed in the outpatient department; he was not concordant with prescribed bronchodilator treatment because the symptoms were mild. He was a smoker of 9 pack-years and continued to smoke. He reported no environmental or occupational exposures to fumes, gas, or dusts. He had no previous history of chest trauma.
On examination the patient was alert and breathless at rest; he showed no signs of respiratory failure, cyanosis, or clubbing. Pulse was 75 beats per min, blood pressure was 145/80 mm Hg, oxygen saturation was 85%, and respiratory rate was 24 breaths per min. On auscultation, breath sounds were diminished in the right lung.
Laboratory investigations showed no abnormalities: no anaemia or erythrocytosis. An electrocardiogram showed no signs of ischaemia, infarction, or ventricular hypertrophy, or abnormalities of rhythm.
A chest x-ray showed a right hydropneumothorax (figure).
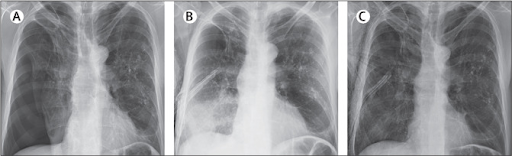
A right chest thoracostomy with a negative pressure suction system was inserted. 1 h later, a repeated chest x-ray showed complete lung expansion with right lower lobe consolidation, and subcutaneous emphysema around the chest tube (figure). In the next 24 h, the patient developed worsening dyspnoea with hypoxaemia; arterial blood gases showed hypocapnia with a pCO2 of 261 mm Hg (normal range 283 387) and hypoxaemia with oxygen saturation of 85% on room air. CT pulmonary angiography ruled out a pulmonary embolism and chest tube malposition but showed right lower lobe consolidation and upper lobe bullae and centrilobular emphysema.
3 days after admission, an additional chest x-ray showed that the consolidation had resolved, with complete lung expansion (figure).
The rapid clearance of the consolidation affecting the collapsed lung led us to a diagnosis of lobar re-expansion pulmonary oedema in the context of a secondary spontaneous pneumothorax.
The patient improved and required no specific therapy. He was allowed home after 7 days; he was well and showed no signs of respiratory distress. He was prescribed tiotropium and brief smoking cessation intervention. He was given an appointment with the stop-smoking service and a test for alpha-1 antitrypsin was booked.
Re-expansion pulmonary oedema (REPE) is a rare complication, likely to occur in less than 1% of all pleural drainage procedures including pneumothorax, haemothorax, and pleural effusion. The mechanism behind REPE is not completely understood but involves an increase in negative intrapleural pressure after rapid draining, which causes a rise in hydrostatic vascular pressure, reperfusion damage, and pulmonary capillary permeability in the atelectatic lung. Pre-existing parenchymal lung illness, or lobar, or segmental bronchial obstruction, prior to pleural drainage, may cause REPE; thus, it is reasonable to postulate that in our patient pre-existing bullae in the upper lobe may have prevented parenchymal collapse and conditioned localised REPE of the lower lobe.
(12). Prof Axel Schambac et al. A new age of precision gene therapy. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01952-9/fulltext
Summary
Gene therapy has become a clinical reality as market-approved advanced therapy medicinal products for the treatment of distinct monogenetic diseases and B-cell malignancies. This Therapeutic Review aims to explain how progress in genome editing technologies offers the possibility to expand both therapeutic options and the types of diseases that will become treatable. To frame these impressive advances in the context of modern medicine, we incorporate examples from human clinical trials into our discussion on how genome editing will complement currently available strategies in gene therapy, which still mainly rely on gene addition strategies. Furthermore, safety considerations and ethical implications, including the issue of accessibility, are addressed as these crucial parameters will define the impact that gene therapy in general and genome editing in particular will have on how we treat patients in the near future.
(13). Sharon C R et al. The cerebroplacental ratio: a new standard diagnostic tool at term gestation to assess fetal risk in labour.
Summary
The cerebroplacental ratio is a Doppler-derived fetal ultrasound metric that can reflect alterations in cerebral blood flow due to fetal hypoxia and increased placental resistance. The cerebroplacental ratio has emerged as a clinical risk assessment tool for placental insufficiency at term gestation in both small for gestational age (SGA) and appropriate for gestational age (AGA) birthweight groups. A key clinical goal before labour onset is to predict fetal intolerance to labour, intrapartum fetal compromise from hypoxia, emergency caesarean delivery, and subsequent serious perinatal morbidity and mortality. Previous studies have associated low cerebroplacental ratios with higher rates of emergency caesarean delivery for fetal distress in labour in both AGA 2 and SGA 3fetuses, and higher rates of stillbirth and perinatal mortality. In a trial evaluating cerebroplacental ratios among both SGA and AGA fetuses, a low cerebroplacental ratio was a better predictor of emergency operative delivery than low birthweight. A randomised trial that screened for cerebroplacental ratio and placental growth factor with recommended planned delivery for abnormal screening versus standard care found no differences in intrapartum interventions or adverse neonatal outcomes. However, the study was probably underpowered, and those who screened positive for a low cerebroplacental ratio had a higher likelihood of operative delivery for fetal distress, meconium, or fetal heart rate abnormalities. Given the promise of cerebroplacental ratios but inconclusive evidence for their clinical integration, a large randomised controlled trial evaluating cerebroplacental ratio screening for delivery decision making was needed.
(14). Elisabeth Mahase. Variability in blood pressure could help predict heart attack and stroke risk, researchers say. BMJ 2024; 384.
Hypertension guidelines should be changed to focus on not just a patient’s blood pressure but how it varies from visit to visit, say researchers from Imperial College London.
(15). Maughan B C, Jarman A F, Redmond A, Geersing G, Kline J A. Pulmonary embolism BMJ 2024; 384 :e071662
What you need to know
Diagnosis of pulmonary embolism (PE) is frequently missed. An estimated 12-36% of patients with PE are misdiagnosed during initial evaluation in emergency departments or primary care clinics
Delayed and missed diagnoses are more common in older adults, in patients with chronic cardiopulmonary disease, those with low pre-test risk of PE, and in patients presenting with cough or gradual onset dyspnoea. Most patients with PE have no symptoms of deep vein thrombosis, and many have no chest pain.
Using D-dimer thresholds adjusted for age or probability may reduce false positive results and rates of computed tomography pulmonary angiography scans.
(15). Smith R. Chatting with the King about cancer. BMJ. 2024 Feb 8;384:q342
King: Good morning. This King Charles. I’m sorry to ring so early, and I’m sure that you’re surprised to hear from me. You’ll know probably that I’ve had cancer diagnosed, and by a strange coincidence while I was waiting in the hospital an aide brought to my attention the blog you posted yesterday about the meaning of cancer.1 As you may guess, I’m a man who likes to think about the big picture of everything, including cancer. That’s why I’m ringing you.
Richard: I’m honoured. The first thing I need to say is that I’m sorry that you’ve had cancer diagnosed. It’s always a shock no matter how much you might be expecting it. The second thing I must say is that my blog was based on a book Making Sense of Cancer: From Its Evolutionary Origin to Its Societal Impact and the Ultimate Solution by Jarle Breivik, a cancer researcher and doctor. He points out that having cancer is tied up with being human and that we can never get rid of cancer without getting rid of our bodies.The book is well worth reading, especially if you are interested in the big picture. Indeed, as you’ve put cancer in the spotlight well, even more in the spotlight you might recommend others to read the book. It hasn’t had the attention it deserves. You might also read and recommend The Emperor of All Maladies: A Biography of Cancer by Siddhartha Mukherjee, an oncologist. He too describes how cancer is us,†and his title shows how in the battle of diseases for pre-eminence cancer wins easily. Your time will be much better spent reading them than listening to lots of witless witterings in the media.
King: I should have more time for reading. Do you mind me asking if you have had cancer?
Richard: Well, I have. and I haven’t. In a literal sense I have because I had a basal cell carcinoma removed from my chest.2 But nobody dies of a basal cell carcinoma, and my cancer was removed within a couple of hours of me having it diagnosed. You’ll know that when people think of cancer they think of the big cancers that can kill you lung, pancreas, bowel, breast, and prostate. Would you mind if I was critical of you?
King: Not at all. A critical friend is the best kind of friend.
Richard: Well, I applaud you for letting the world know you have cancer. An announcement avoids the awful thing of some people knowing, some not knowing, and some knowing and not being sure whether they should do. It also dampens speculation and makes clear that there is nothing unusual about getting cancer: half of us will. But I think that you’ve made a mistake in not announcing the type of cancer. You will know that there are many sorts of cancers, all with different prognoses and treatments, and that they can be at different stages. By not announcing the type you’ve reinforced the misunderstanding that cancer is one disease. You’ve also left open the possibility that you might be either right as rain or dead in a few weeks.
King: I see your point, but you must understand that my advisers, a conservative lot, didn’t want me to say what I had at all. Others have made the same point as you, and we’ll have to think some more. Now, I know you’re not a practising doctor, but have you any thoughts on treatment?
Richard: My first thought is that you should not rush into anything. There is an exaggerated idea that days, even hours matter when treating cancer, but that’s generally not true. Your treatment is likely to be a long haul, and you should take time to consider the best path. You are likely to come under pressure from doctors, family, public opinion, and the media to go for aggressive treatment. You might be thought a wimp if you don’t oh, there’s another book for your reading list: Because Cowards Get Cancer Too by John Diamond. But aggressive treatment may not be the best treatment even if your aim is to put quantity ahead of quality of life. You should talk with a range of doctors and with patients who have opted for different kinds of treatment. My guess is that quality of life will be more important to you than quantity, and you should make your decision accordingly. We know that many people are overtreated at the end of life and as a result spend much of the time they do have in the clutches of doctors, hospitals, operations, and drugs rather than out in the Scottish glens where I suspect you’d rather be.
King: Thank you for that. I’ve cared for nature all my life, and I know that cancer is treated with poisons. Is there a relationship between the treatment of cancer and the environment?
ichard: I know that you have been concerned about the planet, the future of humanity, and justice far longer than most people and you should think of your cancer in that context. There is a good chance that you will have treatment with very expensive drugs, not all of them poisons, which will keep you alive far longer than would have been the case if you developed your cancer even a decade ago. That treatment will add to the damage to the planet through all the carbon consumed in treating you and the waste generated. Many of the plastics and drugs end up in the rivers and sea. Most people in the world will not have access to such treatment. Indeed, many, the majority, don’t even have access to opiates and basic palliative care at the end of life. You’ll read in Jarle Breivik’s book how, despite all the investment in cancer research and treatment, we have more cancer than ever because cancer is a disease of old people and it’s one of the ways we are programmed to die. Clearly a combination of ever more cancer and expensive, environmentally damaging treatments is not sustainable for either the planet or sickness systems.
King: Well thank you. I hadn’t thought of that but will now. I’ll discuss it with my doctors and advisers. I’m interested to find a way to develop something positive out of this bad news. Bye by
(16). Miguel Mansillaet al Symmetric Drug-Related Intertriginous and Flexural Exanthema. February 15, 2024, N Engl J Med 2024; 390:e17,DOI: 10.1056/NEJMicm2310034
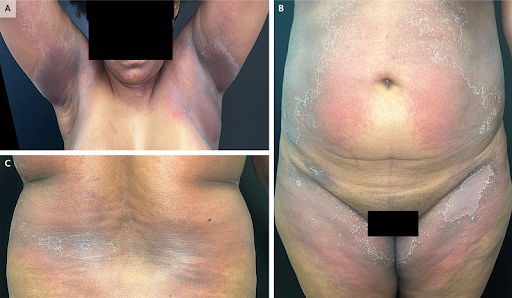
An otherwise healthy 42-year-old woman presented with a 10-day history of a rash in her axillae and on her groin and abdomen. One and a half weeks before the onset of the rash, she had started taking dexketoprofen (a nonsteroidal antiinflammatory drug [NSAID]) at a dose of 25 mg per day to treat knee pain. She reported no fevers, mucosal lesions, or symptoms other than mild pruritus. On physical examination, symmetric patches of reddish-purple skin with peeling borders were present in the cervical and axillary regions (Panel A), the abdominal and inguinal regions (Panel B), and the intertriginous area of the back (Panel C). Owing to the appearance of the rash and the patient’s use of an NSAID, a diagnosis of symmetric drug-related intertriginous and flexural exanthema (SDRIFE) was made. SDRIFE is a drug-induced eruption that is characterized by the presence of symmetric erythema in the intertriginous and flexural areas and by the absence of systemic symptoms. Triggers include the use of beta-lactam agents, macrolides, and NSAIDs. Treatment with a course of topical glucocorticoids was initiated, and discontinuation of dexketoprofen treatment was recommended. One month after the initial presentation, the patient’s rash had abated.
(17). Serena Bagnasco, Renal Malakoplakia. February 17, 2024,,OI: 10.1056/NEJMicm2301676
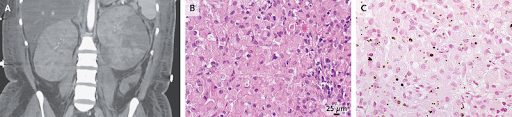
A 33-year-old woman who had been admitted to the hospital for septic shock from Escherichia coli pyelonephritis was noted to have large kidneys on imaging. Computed tomographic imaging of the abdomen showed renal enlargement with heterogeneous decreased perfusion on both sides (Panel A). A subsequent biopsy of the left kidney showed histiocytic infiltration of the renal cortex (Panel B, hematoxylin and eosin staining) and round, basophilic intracytoplasmic inclusions, which are known as Michaelis Gutmann bodies (Panel C, von Kossa calcium staining). A diagnosis of renal malakoplakia was made. Renal malakoplakia is a rare, chronic inflammatory disorder with a pathogenesis that is not well understood. It is thought to be triggered by defective intracellular killing of phagocytosed bacteria, particularly E. coli. It may manifest with recurrent urinary tract infections, which this patient had. The diagnosis is made on the basis of histopathological evaluation, particularly the finding of pathognomonic Michaelis Gutmann bodies (which represent calcified elements of undigested bacteria within macrophages). Although malakoplakia most commonly occurs in immunocompromised patients, this patient had a normal immunologic evaluation. Owing to persistent bacteremia and progressive renal abscesses despite administration of antimicrobial agents, as well as worsening left kidney function, a left nephrectomy was ultimately performed. At follow-up 3 weeks later, the patient was feeling well and had normal renal function.
(18). Seigi Oshima et al. Chylothorax. February 22, 2024, N Engl J Med 2024; 390:e20,DOI: 10.1056/NEJMicm2308973
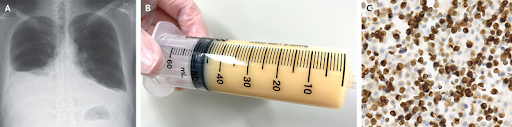
A 63-year-old man with a history of follicular lymphoma presented with a 2-week history of fatigue and 3-day history of dyspnea. The lymphoma had been in remission for 10 months before the current presentation. On physical examination, there were decreased breath sounds at the base of both lungs and no palpable lymphadenopathy. A chest radiograph revealed pleural effusions, which were greater on the right side than on the left (Panel A). In addition, whole-body computed tomography showed diffuse lymphadenopathy and hepatosplenomegaly. Thoracentesis was performed on the right side, and 1 liter of milky, yellow fluid was removed (Panel B). Analysis of the pleural fluid showed a lymphocytic exudate with a triglyceride level of 1225 mg per deciliter (13.8 mmol per liter; reference value, 110 mg per deciliter [1.2 mmol per liter]) and a cholesterol level of 127 mg per deciliter (3.3 mmol per liter; reference value, 250 mg per deciliter [6.5 mmol per liter]). Histopathological analysis of a pleural-fluid cell block showed small, malignant lymphocytes with an immunophenotype that was identical to that seen in the patient’s previous follicular lymphoma (Panel C; immunostain for B-cell lymphoma 2). A diagnosis of chylothorax due to relapsed follicular lymphoma was made. The most common cause of nontraumatic chylothorax is cancer, most frequently lymphoma. Seven days after the initiation of systemic chemotherapy, the pleural effusions had completely abated. After six cycles of treatment, the patient was in partial remission. However, within 1 month, chylothorax and lymphadenopathies recurred. Currently, the patient is undergoing a salvage regimen for relapsed follicular lymphoma, with subsequent chimeric antigen receptor T-cell (CAR-T) therapy planned.
(19). David Dickson et al.Mpox Tongue Lesions. February 24, 2024, DOI: 10.1056/NEJMicm2307920
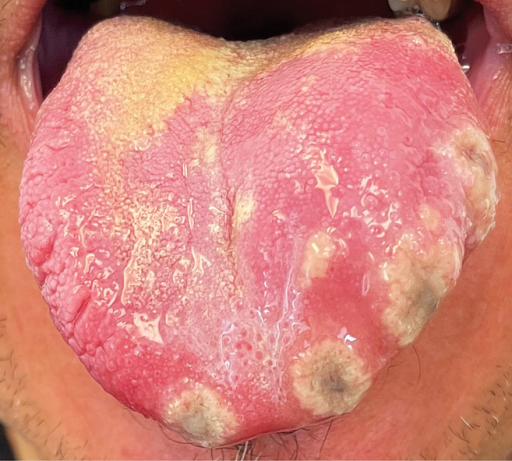
A 49-year-old man with human immunodeficiency virus (HIV) infection presented to a primary care clinic with an 11-day history of painful tongue lesions and a 1-week history of sore throat and fevers. He had last been sexually active with his male partner 9 days before the onset of symptoms; his partner was asymptomatic. Five months before presentation, the patient’s CD4 cell count had been 519 per microliter (reference range, 297 to 1551), and 1 month before presentation, the HIV viral load had been undetectable. On physical examination, four ulcers with central darkening and raised borders were seen on the tip and left lateral aspect of the tongue. Tender submandibular lymphadenopathy was also present on the left side. No other lesions were seen in the mouth or throat or on the skin. Testing of a tongue lesion with a polymerase-chain-reaction assay for the virus that causes mpox (formerly known as monkeypox) was positive. A diagnosis of mpox was made. During the eruptive phase of mpox, a rash is very common, but isolated oral mucosal lesions may be the only mucocutaneous manifestation as occurred in this case. The patient was lost to follow-up with primary care after the diagnosis was made, so no antiviral treatment was given. During a telephone appointment with a different clinic 2 weeks later, he reported feeling in his usual health.
(20). Amrou Sarraj et al. Endovascular thrombectomy plus medical care versus medical care alone for large ischaemic stroke: 1-year outcomes of the SELECT2 trial, The Lancet. 403, 10428, P731-740, 24, 2024
Summary
Background
Multiple randomised trials have shown efficacy and safety of endovascular thrombectomy in patients with large ischaemic stroke. The aim of this study was to evaluate long-term (ie, at 1 year) evidence of benefit of thrombectomy for these patients.
Methods
SELECT2 was a phase 3, open-label, international, randomised controlled trial with blinded endpoint assessment, conducted at 31 hospitals in the USA, Canada, Spain, Switzerland, Australia, and New Zealand. Patients aged 18 85 years with ischaemic stroke due to proximal occlusion of the internal carotid artery or of the first segment of the middle cerebral artery, showing large ischaemic core on non-contrast CT (Alberta Stroke Program Early Computed Tomographic Score of 3 5 [range 0 10, with lower values indicating larger infarctions]) or measuring 50 mL or more on CT perfusion and MRI, were randomly assigned, within 24 h of ischaemic stroke onset, to thrombectomy plus medical care or to medical care alone. The primary outcome for this analysis was the ordinal modified Rankin Scale (range 0 6, with higher scores indicating greater disability) at 1-year follow-up in an intention-to-treat population. The trial is registered at ClinicalTrials.gov (NCT03876457) and is completed.
Findings
The trial was terminated early for efficacy at the 90-day follow-up after 352 patients had been randomly assigned (178 to thrombectomy and 174 to medical care only) between Oct 11, 2019, and Sept 9, 2022. Thrombectomy significantly improved the 1-year modified Rankin Scale score distribution versus medical care alone (Wilcoxon-Mann-Whitney probability of superiority 059 [95% CI 053 064]; p=00019; generalised odds ratio 143 [95% CI 114 178]). At the 1-year follow-up, 77 (45%) of 170 patients receiving thrombectomy had died, compared with 83 (52%) of 159 patients receiving medical care only (1-year mortality relative risk 089 [95% CI 071 111]).
Interpretation
In patients with ischaemic stroke due to a proximal occlusion and large core, thrombectomy plus medical care provided a significant functional outcome benefit compared with medical care alone at 1-year follow-up.
Funding
Stryker Neurovascular.
(21). Layal Chaker et al. Hyperthyroidism. The Lancet. 403, 10428, P768-780, 24, 2024
Summary
Thyrotoxicosis causes a variety of symptoms and adverse health outcomes. Hyperthyroidism refers to increased thyroid hormone synthesis and secretion, most commonly from Graves’ disease or toxic nodular goitre, whereas thyroiditis (typically autoimmune, viral, or drug induced) causes thyrotoxicosis without hyperthyroidism. The diagnosis is based on suppressed serum concentrations of thyroid-stimulating hormone (TSH), accompanied by free thyroxine and total or free tri-iodothyronine concentrations, which are raised (overt hyperthyroidism) or within range (subclinical hyperthyroidism). The underlying cause is determined by clinical assessment, detection of TSH-receptor antibodies and, if necessary, radionuclide thyroid scintigraphy. Treatment options for hyperthyroidism include antithyroid drugs, radioactive iodine, and thyroidectomy, whereas thyroiditis is managed symptomatically or with glucocorticoid therapy. In Graves’ disease, first-line treatment is a 12 18-month course of antithyroid drugs, whereas for goitre, radioactive iodine or surgery are preferred for toxic nodules or goitres. Evidence also supports long-term treatment with antithyroid drugs as an option for patients with Graves’ disease and toxic nodular goitre.
(22). Mitsuhide Narus et al. Targeted molecular medicine: advances in the treatment of metastatic phaeochromocytoma and paraganglioma. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)02828-3/abstract
WHO defines phaeochromocytomas and paragangliomas as neuroendocrine tumours arising from chromaffin cells of the adrenal medulla or extra-adrenal paraganglia, respectively. 1 Since catecholamine-secreting phaeochromocytomas and paragangliomas were first successfully resected 100 years ago, 2 , 3 major advances have occurred in the measurement of catecholamine and catecholamine metabolites, computed cross-sectional imaging, total body nuclear imaging, and laparoscopic approaches to tumour resection. In the early 2000s, many phaeochromocytoma and paraganglioma susceptibility genes were reported, underscoring the frequent hereditary nature of these rare endocrine neoplasms. 4 Concomitantly, metastatic phaeochromocytomas and paragangliomas have been increasingly recognised to be first detected up to 50 years postoperatively. 5 In 2017, WHO’s classification of tumours of endocrine organs advised that all phaeochromocytomas and paragangliomas have malignant potential a potential that is only confirmed when metastatic disease is documented. 1 In the 21st century, the primary diagnostic challenges for phaeochromocytoma and paraganglioma have become the early distinction between metastatic and non-metastatic forms and detecting pathogenic variants in the genes that predispose to the development of phaeochromocytomas and paragangliomas and distant metastases. On the management front, the main challenges remain the detection and treatment of metastatic phaeochromocytoma and paraganglioma, for which curative options are yet to be established.
(23). Ased S M Al et al. Gepotidacin, a new first-in-class antibiotic for treating uncomplicated urinary tract infection. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)02697-1/abstract
Urinary tract infection is the most common bacterial infection affecting humans worldwide and one of the most frequently treated with antibiotics. Women are particularly susceptible to uncomplicated urinary tract infections, with 50 60% of women estimated to have at least one episode of uncomplicated urinary tract infection in their lifetime. 1 In the past decade, antimicrobial resistance has increasingly limited the use of commonly used agents. 2 This issue has been further compounded by the impact of the COVID-19 pandemic 3 on the delivery of primary health care. The difficulty in meeting patients’ demand for appointments coupled with problems in antibiotic supply chains has made it difficult for patients to secure effective treatments for uncomplicated urinary tract infection and has increased the risks of progression to complicated or recurrent urinary tract infections. Within this context, the development of a new oral agent is of both great clinical interest and value.
(24). Vivekanand Tiwari et al. Fever, Rash, and Shortness of Breath in a 69-Year-Old
JAMA. 2024;331(8):698-699. doi:10.1001/jama.2023.25521
A69-year-old man presented to the rheumatology clinic 3 weeks after being hospitalized for fever, fatigue, rash, right periorbital swelling, and shortness of breath. This was his fifth inpatient stay in the past 3 years for similar symptoms. During a recent hospitalization, he had purpuric macules and plaques on his lower extremities bilaterally (Figure, left panel). Laboratory testing revealed a white blood cell count of 2.9 × 103/L (reference, 4.2-10.8 ×103/L); mean corpuscular volume, 96.1 fL (reference, 80-96 fL); hemoglobin level, 12.4 mg/dL (reference, 14.0-18.0 mg/dL); and a normal platelet count. C-reactive protein level was 270 mg/L (reference, 0-5 mg/L) and erythrocyte sedimentation rate was 98 mm/h (reference, 0-20 mm/h).Results of testing for antinuclear antibody and antineutrophil cytoplasmic autoantibodies were negative. Computed tomography of the chest revealed numerous small, bilateral pulmonary nodules (Figure, right panel). Magnetic resonance imaging of the brain revealed bilateral inferior rectus muscle enlargement with mild inflammatory changes along the extraocular muscles and optic nerves. A skin biopsy showed a perivascular neutrophilic infiltrate consistent with leukocytoclastic vasculitis. During his most recent hospitalization, he was treated with intravenous methylprednisolone (40 mg twice daily) for 5 days and discharged taking oral prednisone (40 mg daily) for 4 weeks. Previously, he had pericardial and pleural effusions and pulmonary infiltrates. Pericardial biopsy showed organizing pericarditis and lung biopsy revealed cryptogenic organizing pneumonia, with negative IgG4 stains. Results of bone marrow biopsy, including flow cytometry and cytogenetic testing, performed 18 months prior to the current presentation were normal.
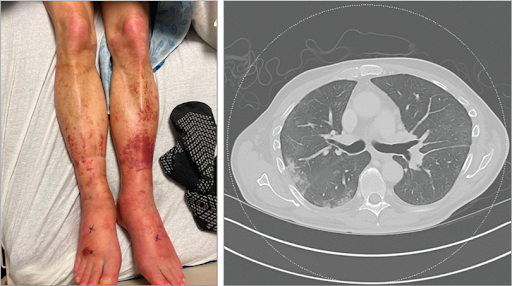
At presentation, his temperature was 36.4 °C (97.5 °F); blood pressure, 149/66 mm Hg; heart rate, 90/min; and oxygen saturation, 98% on room air. Physical examination findings were normal.
What Would You Do Next?
Check serum concentration of interleukin 18 (IL-18)
Measure plasma adenosine deaminase 2 (ADA2) activity
Order serum anticollagen type II antibody levels
Perform testing for ubiquitin-like modifier activating enzyme 1 (UBA1) gene variants
Diagnosis
VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome
What to Do Next
D. Perform testing for ubiquitin-like modifier activating enzyme 1 (UBA1) gene variants.
The key to the correct diagnosis is recognizing that recurrent episodes of fever and inflammation affecting skin, lungs, and other organs, and macrocytic anemia, are characteristic of VEXAS syndrome. Choice A is not recommended because IL-18 is a nonspecific marker of inflammation. Choice B is incorrect because this patient did not have peripheral vasculopathy and stroke, which are characteristic of ADA2 deficiency. Choice C would not be useful because anticollagen II antibody levels are elevated in several inflammatory diseases.
Discussion
First reported in 2020, VEXAS syndrome is a rare disease caused by a UBA1 gene variant in hematopoietic progenitor cells.1 UBA1 gene variants reduce function of the UBA1 enzyme, which facilitates protein ubiquitination, a posttranslational modification process essential for cell signaling and protein degradation. Patients with VEXAS syndrome experience autoinflammation, characterized by recurrent episodes of inflammation in various organs.2
UBA1 gene variants are X-linked, so VEXAS syndrome has been reported only in men, or in women with monosomy of the X chromosome (Turner syndrome).3 As a somatic gene variant, it is not inherited, and VEXAS syndrome predominantly affects people older than 50 years.4 The prevalence of VEXAS syndrome is uncertain. A recent study of exome sequencing on DNA extracted from peripheral blood reported that 1 in 4269 men older than 50 years had a UBA1 variant associated with VEXAS syndrome, although not all patients had symptoms of VEXAS syndrome.4
Clinical manifestations of VEXAS syndrome are variable and include skin lesions (eg, cutaneous vasculitis, neutrophilic dermatoses, erythema nodosum), recurrent fever, weight loss, orchitis, hepatosplenomegaly, ocular manifestations (eg, orbital inflammation, uveitis, scleritis, episcleritis), sensorineural hearing loss, and inflammatory arthritis.2,5 Approximately 40% to 60% of patients develop thromboembolism, and up to 77% have pulmonary infiltrates on computed tomography scans.6 Patients with VEXAS syndrome also have frequently been diagnosed with Sweet syndrome, giant cell arteritis, polyarteritis nodosa, relapsing polychondritis, myelodysplastic syndromes, and multiple myeloma.1-3,5 VEXAS syndrome should be considered in men with late-onset, undiagnosed inflammatory syndromes with associated hematologic abnormalities.
Elevated inflammatory markers (C-reactive protein level, erythrocyte sedimentation rate) and macrocytic anemia are typical laboratory findings. Lymphopenia occurs in approximately 80% and thrombocytopenia in 50% of patients with VEXAS syndrome.2 On bone marrow biopsy, more than 10% cytoplasmic vacuoles in myeloid and erythroid precursor cells is an indication for UB1A testing.2 The diagnosis of VEXAS syndrome is made by identifying UBA1 gene variants with polymerase chain reaction based sequencing of DNA extracted from peripheral blood, bone marrow, or formalin-fixed paraffin-embedded tissue in a patient with symptoms characteristic of VEXAS syndrome.7,8
In the absence of rigorous trials, corticosteroids are typically first-line treatment. Trials of steroid-sparing agents such as tocilizumab, azacitidine, Janus kinase (JAK) inhibitors, and allogeneic stem cell transplant have included very small numbers of patients and have produced variable results with high rates of adverse events (eg, pneumonia, thromboembolism). Therefore, treatment should be individualized.9,10
Patients diagnosed with VEXAS syndrome have a 5-year survival of approximately 63%,6 and those with the p.Met41Val variant had a median survival of 9 years after symptom onset.8
Patient Outcome
A polymerase chain reaction based next-generation sequencing of DNA from the patient’s prior bone marrow specimen identified a pathogenic UBA1 gene variant, UBA1 c.121A>G (p.Met41Val), confirming the diagnosis of VEXAS syndrome. In addition to prednisone (20 mg) daily, the patient was prescribed weekly subcutaneous tocilizumab (162 mg), which was discontinued after 4 months due to leukopenia and recurrent cutaneous vasculitis on his legs. The patient did not want to enroll in a clinical trial and declined evaluation for allogeneic hematopoietic stem cell transplantation. Ten months after presentation, he was taking prednisone (15 mg) daily and his physical examination findings were normal.
(25). Shiyao Wang et al, February 29, 2024. Crazy-Paving Pattern in Pulmonary Sarcoidosis. N Engl J Med 2024; 390:e21,DOI: 10.1056/NEJMicm2308650
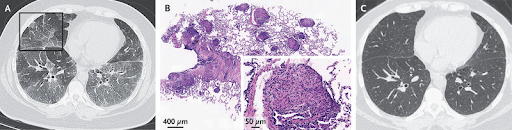
A 28-year-old woman presented to the hospital with a 6-month history of dry cough. She was a lifetime nonsmoker and reported having no fevers, joint aches, eye pain, or rashes. On physical examination, auscultation of both lower lungs revealed fine crackles. High-resolution computed tomography (CT) of the chest showed mediastinal lymphadenopathy and diffuse ground-glass opacities (Panel A, axial view). Also visible were areas of superimposed interlobular and intralobular septal thickening, a pattern known as crazy paving because of the resemblance to irregular paving stones (Panel A, box). The serum level of angiotensin-converting enzyme was 80 U per liter (reference range, 17 to 55). A subsequent transbronchial lung biopsy showed multiple noncaseating granulomas (Panel B, inset showing granuloma; hematoxylin and eosin stain). Bronchoalveolar-lavage cultures, histopathological analysis, and molecular testing were negative for infectious organisms, including Mycobacterium tuberculosis. A diagnosis of pulmonary sarcoidosis was made. The crazy-paving pattern is commonly associated with pulmonary alveolar proteinosis, but it is also seen in a variety of other conditions, such as acute respiratory distress syndrome, Pneumocystis jirovecii pneumonia, and sarcoidosis, as in this case. A tapering dose of prednisone was initiated. By 6 months after the initiation of treatment, the patient’s symptoms had abated and the radiographic findings had greatly improved (Panel C). At that point, prednisone was discontinued, and the patient’s condition stabilized. The associated symptoms did not recur, and repeat chest CT after 1 year of follow-up did not suggest progression of the lesions.
(26). Robert A. Wood, February 25, 2024. Omalizumab for the Treatment of Multiple Food Allergies
Abstract
Background
Food allergies are common and are associated with substantial morbidity; the only approved treatment is oral immunotherapy for peanut allergy.
Methods
In this trial, we assessed whether omalizumab, a monoclonal anti-IgE antibody, would be effective and safe as monotherapy in patients with multiple food allergies. Persons 1 to 55 years of age who were allergic to peanuts and at least two other trial-specified foods (cashew, milk, egg, walnut, wheat, and hazelnut) were screened. Inclusion required a reaction to a food challenge of 100 mg or less of peanut protein and 300 mg or less of the two other foods. Participants were randomly assigned, in a 2:1 ratio, to receive omalizumab or placebo administered subcutaneously (with the dose based on weight and IgE levels) every 2 to 4 weeks for 16 to 20 weeks, after which the challenges were repeated. The primary end point was ingestion of peanut protein in a single dose of 600 mg or more without dose-limiting symptoms. The three key secondary end points were the consumption of cashew, of milk, and of egg in single doses of at least 1000 mg each without dose-limiting symptoms. The first 60 participants (59 of whom were children or adolescents) who completed this first stage were enrolled in a 24-week open-label extension.
Results
Of the 462 persons who were screened, 180 underwent randomization. The analysis population consisted of the 177 children and adolescents (1 to 17 years of age). A total of 79 of the 118 participants (67%) receiving omalizumab met the primary end-point criteria, as compared with 4 of the 59 participants (7%) receiving placebo (P0.001). Results for the key secondary end points were consistent with those of the primary end point (cashew, 41% vs. 3%; milk, 66% vs. 10%; egg, 67% vs. 0%; P0.001 for all comparisons). Safety end points did not differ between the groups, aside from more injection-site reactions in the omalizumab group.
Conclusions
In persons as young as 1 year of age with multiple food allergies, omalizumab treatment for 16 weeks was superior to placebo in increasing the reaction threshold for peanut and other common food allergens. (Funded by the National Institute of Allergy and Infectious Diseases and others).

