Journal scan: A review of 40 recent papers of immediate clinical significance, harvested from major international journals
From the desk of the Editor-in-Chief
[1]. Reinoud E. Knops et al. A Dual-Chamber Leadless Pacemaker. N Engl J Med. 2023 Jun 22;388(25):2360-2370.
Background
Single-chamber ventricular leadless pacemakers do not support atrial pacing or consistent atrioventricular synchrony. A dual-chamber leadless pacemaker system consisting of two devices implanted percutaneously, one in the right atrium and one in the right ventricle, would make leadless pacemaker therapy a treatment option for a wider range of indications.
Methods
We conducted a prospective, multicenter, single-group study to evaluate the safety and performance of a dual-chamber leadless pacemaker system. Patients with a conventional indication for dual-chamber pacing were eligible for participation. The primary safety end point was freedom from complications (i.e., device- or procedure-related serious adverse events) at 90 days. The first primary performance end point was a combination of adequate atrial capture threshold and sensing amplitude at 3 months. The second primary performance end point was at least 70% atrioventricular synchrony at 3 months while the patient was sitting.
Results
Among the 300 patients enrolled, 190 (63.3%) had sinus-node dysfunction and 100 (33.3%) had atrioventricular block as the primary pacing indication. The implantation procedure was successful (i.e., two functioning leadless pacemakers were implanted and had established implant-to-implant communication) in 295 patients (98.3%). A total of 35 device- or procedure-related serious adverse events occurred in 29 patients. The primary safety end point was met in 271 patients (90.3%; 95% confidence interval [CI], 87.0 to 93.7), which exceeded the performance goal of 78% (P<0.001). The first primary performance end point was met in 90.2% of the patients (95% CI, 86.8 to 93.6), which exceeded the performance goal of 82.5% (P<0.001). The mean (±SD) atrial capture threshold was 0.82±0.70 V, and the mean P-wave amplitude was 3.58±1.88 mV. Of the 21 patients (7%) with a P-wave amplitude of less than 1.0 mV, none required device revision for inadequate sensing. At least 70% atrioventricular synchrony was achieved in 97.3% of the patients (95% CI, 95.4 to 99.3), which exceeded the performance goal of 83% (P<0.001).
Conclusions
The dual-chamber leadless pacemaker system met the primary safety end point and provided atrial pacing and reliable atrioventricular synchrony for 3 months after implantation.
[2]. Surya P. Bhatt, et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. 2023.
Background
In some patients with chronic obstructive pulmonary disease (COPD), type 2 inflammation may increase exacerbation risk and may be indicated by elevated blood eosinophil counts. Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and interleukin-13, key drivers of type 2 inflammation.
Methods
In a phase 3, double-blind, randomized trial, we assigned patients with COPD who had a blood eosinophil count of at least 300 per microliter and an elevated exacerbation risk despite the use of standard triple therapy to receive dupilumab (300 mg) or placebo subcutaneously once every 2 weeks. The primary end point was the annualized rate of moderate or severe exacerbations of COPD. Key secondary and other end points that were corrected for multiplicity were the change in the prebronchodilator forced expiratory volume in 1 second (FEV1) and in the scores on the St. Georges Respiratory Questionnaire (SGRQ; range, 0 to 100, with lower scores indicating a better quality of life) and the Evaluating Respiratory Symptoms in COPD (E-RSCOPD; range, 0 to 40, with lower scores indicating less severe symptoms).
Results
A total of 939 patients underwent randomization: 468 to the dupilumab group and 471 to the placebo group. The annualized rate of moderate or severe exacerbations was 0.78 (95% confidence interval [CI], 0.64 to 0.93) with dupilumab and 1.10 (95% CI, 0.93 to 1.30) with placebo (rate ratio, 0.70; 95% CI, 0.58 to 0.86; P<0.001). The prebronchodilator FEV1 increased from baseline to week 12 by a least-squares (LS) mean of 160 ml (95% CI, 126 to 195) with dupilumab and 77 ml (95% CI, 42 to 112) with placebo (LS mean difference, 83 ml; 95% CI, 42 to 125; P<0.001), a difference that was sustained through week 52. At week 52, the SGRQ score had improved by an LS mean of 9.7 (95% CI, 11.3 to 8.1) with dupilumab and 6.4 (95% CI, 8.0 to 4.8) with placebo (LS mean difference, 3.4; 95% CI, 5.5 to 1.3; P=0.002). The E-RSCOPD score at week 52 had improved by an LS mean of 2.7 (95% CI, 3.2 to 2.2) with dupilumab and 1.6 (95% CI, 2.1 to 1.1) with placebo (LS mean difference, 1.1; 95% CI, 1.8 to 0.4; P=0.001). The numbers of patients with adverse events that led to discontinuation of dupilumab or placebo, serious adverse events, and adverse events that led to death were balanced in the two groups.
Conclusions
Among patients with COPD who had type 2 inflammation as indicated by elevated blood eosinophil counts, those who received dupilumab had fewer exacerbations, better lung function and quality of life, and less severe respiratory symptoms than those who received placebo.
[3]. Pablo Garcia-Pavia, et al. Phase 1 Trial of Antibody NI006 for Depletion of Cardiac Transthyretin Amyloid. 2023
Background
Transthyretin amyloid (ATTR) cardiomyopathy is a progressive and fatal disease caused by misfolded transthyretin. Despite advances in slowing disease progression, there is no available treatment that depletes ATTR from the heart for the amelioration of cardiac dysfunction. NI006 is a recombinant human anti-ATTR antibody that was developed for the removal of ATTR by phagocytic immune cells.
Methods
In this phase 1, double-blind trial, we randomly assigned (in a 2:1 ratio) 40 patients with wild-type or variant ATTR cardiomyopathy and chronic heart failure to receive intravenous infusions of either NI006 or placebo every 4 weeks for 4 months. Patients were sequentially enrolled in six cohorts that received ascending doses (ranging from 0.3 to 60 mg per kilogram of body weight). After four infusions, patients were enrolled in an open-label extension phase in which they received eight infusions of NI006 with stepwise increases in the dose. The safety and pharmacokinetic profiles of NI006 were assessed, and cardiac imaging studies were performed.
Results
The use of NI006 was associated with no apparent drug-related serious adverse events. The pharmacokinetic profile of NI006 was consistent with that of an IgG antibody, and no antidrug antibodies were detected. At doses of at least 10 mg per kilogram, cardiac tracer uptake on scintigraphy and extracellular volume on cardiac magnetic resonance imaging, both of which are imaging-based surrogate markers of cardiac amyloid load, appeared to be reduced over a period of 12 months. The median N-terminal proB-type natriuretic peptide and troponin T levels also seemed to be reduced.
Conclusions
In this phase 1 trial of the recombinant human antibody NI006 for the treatment of patients with ATTR cardiomyopathy and heart failure, the use of NI006 was associated with no apparent drug-related serious adverse events.
[4]. Meanie, R, et al. Temporal Trends in the Use of Computed Tomographic Pulmonary Angiography for Suspected Pulmonary Embolism in the Emergency Department. Ann Intern Med. 2023 Jun;176(6):761-768.
Background
Recently, validated clinical decision rules have been developed that avoid unnecessary use of computed tomographic pulmonary angiography (CTPA) in patients with suspected pulmonary embolism (PE) in the emergency department (ED).
Objective:
To measure any resulting change in CTPA use for suspected PE.
Design:
Retrospective analysis.
Setting:
26 European EDs in 6 countries.
Patients:
Patients with CTPA performed for suspected PE in the ED during the first 7days of each odd month between January 2015 and December 2019.
Measurements:
The primary end points were the CTPAs done for suspected PE in the ED and the number of PEs diagnosed in the ED each year adjusted to an annual census of 100000 ED visits. Temporal trends were estimated using generalized linear mixed regression models.
Results:
8970 CTPAs were included (median age, 63 years; 56% female). Statistically significant temporal trends for more frequent use of CTPA (836 per 100 000 ED visits in 2015 vs. 1112 in 2019; P < 0.001), more diagnosed PEs (138 per 100 000 in 2015 vs. 164 in 2019; P =0.028), a higher proportion of low-risk PEs (annual percent change [APC], 13.8% [95% CI, 2.6% to 30.1%]) with more ambulatory management (APC, 19.3% [CI, 4.1% to 45.1%]), and a lower proportion of intensive care unit admissions (APC, 8.9% [CI, 17.1% to 0.3%]) were observed.
Limitation:
Data were limited to 7 days every 2 months.
Conclusion:
Despite the recent validation of clinical decision rules to limit the use of CTPA, an increase in the CTPA rate along with more diagnosed PEs and especially low-risk PEs were instead observed.
[5]. Wayne A. Ray, et al. Risk for Bleeding-Related Hospitalizations During Use of Amiodarone With Apixaban or Rivaroxaban in Patients With Atrial Fibrillation. A Retrospective Cohort Study. Ann Intern Med. 2023 Jun;176(6):
Background:
Amiodarone, the most effective antiarrhythmic drug in atrial fibrillation, inhibits apixaban and rivaroxaban elimination, thus possibly increasing anticoagulant-related risk for bleeding.
Objective:
For patients receiving apixaban or rivaroxaban, to compare risk for bleeding-related hospitalizations during treatment with amiodarone versus flecainide or sotalol, antiarrhythmic drugs that do not inhibit these anticoagulants elimination.
Design:
Retrospective cohort study.
Setting:
U.S. Medicare beneficiaries aged 65 years or older.
Patients:
Patients with atrial fibrillation began anticoagulant use between 1 January 2012 and 30 November 2018 and subsequently initiated treatment with study antiarrhythmic drugs.
Measurements:
Time to event for bleeding-related hospitalizations (primary outcome) and ischemic stroke, systemic embolism, and death with or without recent (past 30 days) evidence of bleeding (secondary outcomes), adjusted with propensity score overlap weighting.
Results:
There were 91 590 patients (mean age, 76.3 years; 52.5% female) initiating use of study anticoagulants and antiarrhythmic drugs, 54 977 with amiodarone and 36 613 with flecainide or sotalol. Risk for bleeding-related hospitalizations increased with amiodarone use (rate difference [RD], 17.5 events [95% CI, 12.0 to 23.0 events] per 1000 person-years; hazard ratio [HR], 1.44 [CI, 1.27 to 1.63]). Incidence of ischemic stroke or systemic embolism did not increase (RD, 2.1 events [CI, 4.7 to 0.4 events] per 1000 person-years; HR, 0.80 [CI, 0.62 to 1.03]). The risk for death with recent evidence of bleeding (RD, 9.1 events [CI, 5.8 to 12.3 events] per 1000 person-years; HR, 1.66 [CI, 1.35 to 2.03]) was greater than that for other deaths (RD, 5.6 events [CI, 0.5 to 10.6 events] per 1000 person-years; HR, 1.15 [CI, 1.00 to 1.31]) (HR comparison: P = 0.003). The increased incidence of bleeding-related hospitalizations for rivaroxaban (RD, 28.0 events [CI, 18.4 to 37.6 events] per 1000 person-years) was greater than that for apixaban (RD, 9.1 events [CI, 2.8 to 15.3 events] per 1000 person-years) (P = 0.001).
Limitation:
Possible residual confounding.
Conclusion:
In this retrospective cohort study, patients aged 65 years or older with atrial fibrillation treated with amiodarone during apixaban or rivaroxaban use had greater risk for bleeding-related hospitalizations than those treated with flecainide or sotalol.
Primary Funding Source:
National Heart, Lung, and Blood Institute.
[6]. Marika M. Cusick et al. Population-Wide Screening for Chronic Kidney Disease, A Cost-Effectiveness Analysis. Ann Intern Med. 2023 Jun;176(6):788-797.
Background:
Sodiumglucose cotransporter-2 (SGLT2) inhibitors have the potential to alter the natural history of chronic kidney disease (CKD), and they should be included in cost-effectiveness analyses of screening for CKD.
Objective:
To determine the cost-effectiveness of adding population-wide screening for CKD.
Design:
Markov cohort model.
Data Sources:
NHANES (National Health and Nutrition Examination Survey), U.S. Centers for Medicare & Medicaid Services data, cohort studies, and randomized clinical trials, including the DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) trial.
Target Population:
Adults.
Time Horizon:
Lifetime.
Perspective:
Health care sector.
Intervention:
Screening for albuminuria with and without adding SGLT2 inhibitors to the current standard of care for CKD.
Outcome Measures:
Costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs), all discounted at 3% annually.
Results of Base-Case Analysis:
One-time CKD screening at age 55 years had an ICER of $86300 per QALY gained by increasing costs from $249800 to $259000 and increasing QALYs from 12.61 to 12.72; this was accompanied by a decrease in the incidence of kidney failure requiring dialysis or kidney transplant of 0.29 percentage points and an increase in life expectancy from 17.29 to 17.45 years. Other options were also cost-effective. During ages 35 to 75 years, screening once prevented dialysis or transplant in 398000 people and screening every 10 years until age 75 years cost less than $100000 per QALY gained.
Results of Sensitivity Analysis:
When SGLT2 inhibitors were 30% less effective, screening every 10 years during ages 35 to 75 years cost between $145400 and $182600 per QALY gained, and price reductions would be required for screening to be cost-effective.
Limitation:
The efficacy of SGLT2 inhibitors was derived from a single randomized controlled trial.
Conclusion:
Screening adults for albuminuria to identify CKD could be cost-effective in the United States.
Primary Funding Source:
Agency for Healthcare Research and Quality, Veterans Affairs Office of Academic Affiliations, and National Institute of Diabetes and Digestive and Kidney Diseases.
[7]. William B. Feldman. Chronic Obstructive Pulmonary Disease Exacerbations and Pneumonia Hospitalizations Among New Users of Combination Maintenance Inhalers. , JAMA Intern Med. 2023.
Question
In patients with chronic obstructive pulmonary disase (COPD), are combination inhalers containing long-acting muscarinic antagonists (LAMAs) and long-acting β-agonists (LABAs) associated with a reduced incidence of exacerbations and pneumonia hospitalizations compared with inhalers containing inhaled corticosteroids (ICSs) and LABAs?
Findings
In this 1:1 propensity scorematched cohort study of 60 432 patients with COPD in a large US commercial insurance claims database, LAMA-LABA therapy was associated with an 8% reduction in the rate of first moderate or severe COPD exacerbation and a 20% reduction in the rate of first pneumonia hospitalization compared with ICS-LABA therapy.
Meaning
The results of this study suggest that LAMA-LABA therapy should be preferred to ICS-LABA for patients with COPD.
Importance
Clinical guidelines on chronic obstructive pulmonary disease (COPD) recommend inhalers containing long-acting muscarinic antagonists (LAMAs) and long-acting β-agonists (LABAs) over inhalers containing inhaled corticosteroids (ICSs) and LABAs. However, data from randomized clinical trials comparing these combination inhalers (LAMA-LABAs vs ICS-LABAs) have been conflicting and raised concerns of generalizability.
Objective
To assess whether LAMA-LABA therapy is associated with reduced COPD exacerbations and pneumonia hospitalizations compared with ICS-LABA therapy in routine clinical practice.
Design, Setting, and Participants
This was a 1:1 propensity scorematched cohort study using Optums Clinformatics Data Mart, a large commercial insuranceclaims database. Patients must have had a diagnosis of COPD and filled a new prescription for a combination LAMA-LABA or ICS-LABA inhaler between January 1, 2014, and December 31, 2019. Patients younger than 40 years were excluded, as were those with a prior diagnosis of asthma. The current analysis was performed from February 2021 to March 2023.
Exposures
Combination LAMA-LABA inhalers (aclidinium-formoterol, glycopyrronium-formoterol, glycopyrronium-indacaterol, tiotropium-olodaterol, or umeclidinium-vilanterol) and combination ICS-LABA inhalers (budesonide-formoterol, fluticasone-salmeterol, fluticasone-vilanterol, or mometasone-formoterol).
Main Outcome
The primary effectiveness outcome was first moderate or severe COPD exacerbation, and the primary safety outcome was first pneumonia hospitalization. Propensity score matching was used to control for confounding between the 2 groups. Logistic regression analysis was used to estimate propensity scores. Hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards models stratified on matched pairs.
Results
Among 137 833 patients (mean [SD] age, 70.2 [9.9] years; 69 530 [50.4%] female) (107 004 new ICS-LABA users and 30 829 new LAMA-LABA users), 30 216 matched pairs were identified for the primary analysis. Compared with ICS-LABA use, LAMA-LABA use was associated with an 8% reduction in the rate of first moderate or severe COPD exacerbation (HR, 0.92; 95% CI, 0.89-0.96) and a 20% reduction in the rate of first pneumonia hospitalization (HR, 0.80; 95% CI, 0.75-0.86). These findings were robust across a range of prespecified subgroup and sensitivity analyses.
Conclusion
In this cohort study, LAMA-LABA therapy was associated with improved clinical outcomes compared with ICS-LABA therapy, suggesting that LAMA-LABA therapy should be preferred for patients with COPD.
[8]. Deborah J. Wexler et al. Comparative Effects of Glucose-Lowering Medications on Kidney Outcomes in Type 2 Diabetes, The GRADE Randomized Clinical Trial. JAMA Intern Med. 2023
Question
Do glucose-lowering medications have different effects on kidney outcomes?
Findings
In this randomized clinical trial including 5047 patients with type 2 diabetes, those receiving metformin treatment and predominantly without kidney disease at baseline were randomly assigned to treatment with a sulfonylurea, a dipeptidyl peptidase 4 inhibitor, a glucagonlike peptide 1 receptor agonist, or basal insulin; all groups had good glycemic and blood pressure management. There were no significant differences in decreased estimated glomerular filtration rate, progression of albuminuria, dialysis, kidney transplant, or death during 5 years of follow-up.
Meaning
In patients with type 2 diabetes treated with metformin, kidney outcomes do not appear to differ by treatment with 1 of 4 second glucose-lowering medication classes evaluated in this randomized clinical trial.
Importance
Type 2 diabetes (T2D) is the leading cause of kidney disease in the US. It is not known whether glucose-lowering medications differentially affect kidney function.
Objective
To evaluate kidney outcomes in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness (GRADE) trial comparing 4 classes of glucose-lowering medications added to metformin for glycemic management in individuals with T2D.
Design, Setting, and Participants
A randomized clinical trial was conducted at 36 sites across the US. Participants included adults with T2D for less than 10 years, a hemoglobin A1c level between 6.8% and 8.5%, and estimated glomerular filtration rate (eGFR) greater than or equal to 60 mL/min/1.73 m2 who were receiving metformin treatment. A total of 5047 participants were enrolled between July 8, 2013, and August 11, 2017, and followed up for a mean of 5.0 years (range, 0-7.6 years). Data were analyzed from February 21, 2022, to March 27, 2023.
Interventions
Addition of insulin glargine, glimepiride, liraglutide, or sitagliptin to metformin, with the medication combination continued until the HbA1c was greater than 7.5%; thereafter, insulin was added to maintain glycemic control.
Main Outcomes and Measures
Chronic eGFR slope (change in eGFR between year 1 and trial end) and a composite kidney disease progression outcome (albuminuria, dialysis, transplant, or death due to kidney disease). Secondary outcomes included incident eGFR less than 60 mL/min/1.73 m2, 40% decrease in eGFR to less than 60 mL/min/1.73 m2, doubling of urine albumin-to-creatinine ratio (UACR) to 30 mg/g or greater, and progression of Kidney Disease Improving Global Outcomes stage. Analyses were intention-to-treat.
Results
Of the 5047 participants, 3210 (63.6%) were men. Baseline characteristics were mean (SD) age 57.2 (10.0) years; HbA1c 7.5% (0.5%); diabetes duration, 4.2 (2.7) years; body mass index, 34.3 (6.8); blood pressure 128.3/77.3 (14.7/9.9) mm Hg; eGFR 94.9 (16.8) mL/min/1.73 m2; and median UACR, 6.4 (IQR 3.1-16.9) mg/g; 2933 (58.1%) were treated with renin-angiotensin-aldosterone inhibitors. Mean chronic eGFR slope was 2.03 (95% CI, 2.20 to 1.86) mL/min/1.73 m2 per year for patients receiving sitagliptin; glimepiride, 1.92 (95% CI, 2.08 to 1.75) mL/min/1.73 m2 per year; liraglutide, 2.08 (95% CI, 2.26 to 1.90) mL/min/1.73 m2 per year; and insulin glargine, 2.02 (95% CI, 2.19 to 1.84) mL/min/1.73 m2 per year (P=.61). Mean composite kidney disease progression occurred in 135 (10.6%) patients receiving sitagliptin; glimepiride, 155 (12.4%); liraglutide, 152 (12.0%); and insulin glargine, 150 (11.9%) (P=.56). Most of the composite outcome was attributable to albuminuria progression (98.4%). There were no significant differences by treatment assignment in secondary outcomes. There were no adverse kidney events attributable to medication assignment.
Conclusions and Relevance
In this randomized clinical trial, among people with T2D and predominantly free of kidney disease at baseline, no significant differences in kidney outcomes were observed during 5 years of follow-up when a dipeptidyl peptidase 4 inhibitor, sulfonylurea, glucagonlike peptide 1 receptor agonist, or basal insulin was added to metformin for glycemic control.
[9]. Michael P. Lavelle, et al. Wide Complex Tachycardia Observed During Pregnancy, JAMA Intern Med. 2023.
A pregnant woman with no significant medical history presented to an outpatient cardiology clinic for evaluation of intermittent, transient but rapid palpitations, some with momentary light-headedness. None lasted more than about 20 seconds, and there were no syncopal events. At no time did she note chest pain or dyspnea. There was no family history of dysrhythmia. There was no evidence of structural heart disease on physical examination, during which blood pressure was normal, and her pulse rate was 78/min. Baseline laboratory values were unremarkable, as was an echocardiogram. She was referred for a 24-hour Holter monitor. She was taking no medications, except prenatal vitamins. She had no history of smoking or recreational drug abuse, but drank 3 cups of coffee per day. Findings from the monitor are shown.
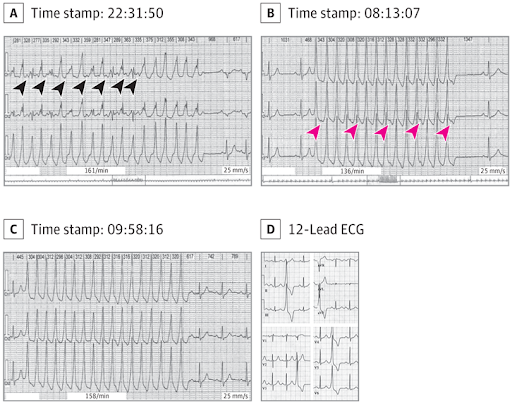
A, Competing sinus tachycardia and ventricular tachycardia is shown. On careful observation, P waves (black arrowheads) can be seen just before fusion beats of sinus activity and the ventricular tachycardia. B, P waves (red arrowheads) are observed, demonstrating atrioventricular dissociation. C, P waves demonstrating atrioventricular dissociation are also noted here and are consistent with nonsustained ventricular tachycardia. In A through C, each strip is 8 seconds long. D, Sections from a baseline 12-lead ECG reveal morphology of frequent premature ventricular contractions in each lead. These localize to the right ventricular outflow tract (left bundle-branch block morphology and inferior frontal axis).
Questions: How do you interpret these Holter monitor strips? What is the most likely etiology of the tachycardia?
Interpretation and Discussion
Review of the second and third Holter monitor strips (Figure, B and C) demonstrates monomorphic wide complex tachycardia (WCT), approximately 200/min, with evidence of atrioventricular dissociation, as P waves are seen marching through at a slower rate (approximately 60/min). Also, the tachyarrhythmia begins without any prior P wave (no change in the preceding QRS or T wave to suggest a hidden P wave). These findings support nonsustained ventricular tachycardia (NSVT) as the mechanism of the WCT. The first Holter monitor strip (Figure, A) demonstrates a WCT at approximately 180/min with at least 2 distinct QRS morphologies with an alternating pattern. On careful observation, P waves are visible before every other complex, with a rate of around 100/min. This is consistent with simultaneous NSVT and sinus tachycardia with variable degrees of fusion of the QRS complexes, explaining the multiple QRS morphologies. At the onset of a WCT, a beat with an intermediate morphology between the baseline and tachycardia QRS morphology can represent fusion from ventricular tachycardia (VT) or progressive aberrancy in the setting of supraventricular tachycardia (SVT) (had SVT been the case).1 In true fusion of sinus rhythm and VT, the observed PR interval is shorter than the PR interval at baseline, as is seen here. On the other hand, in the setting of SVT with aberrant conduction, it would be equal to or longer than the baseline PR interval. Both of the findings support the VT and fusion that are observed in the patients strip.
Further evaluation of the patient via 12-lead electrocardiogram (Figure, D) captured frequent monomorphic premature ventricular contractions (PVCs) with a morphology in all 12 leads resembling that of her NSVT. The most common type of idiopathic ventricular arrhythmias observed in young patients are outflow tract ventricular arrhythmias, with the vast majority (70%-80%) originating from the right ventricular outflow tract (RVOT)2; structural cardiac disorders are most commonly not evident. Several features of the patients PVC localize to the RVOT on the 12-lead electrocardiogram (namely, a left bundle-branch block morphology and inferior frontal axis). With regard to the management of symptomatic RVOT PVC and NSVT, first-line medical therapy includes treatment with β-blockers, followed, if necessary, by treatment with antiarrhythmic drugs and/or catheter ablation. At the time of the patients presentation, she was pregnant and did not want to pursue invasive diagnostic or therapeutic interventions. For this reason, an electrophysiology study and ablation was deferred, and she was medically managed with β-blockade. The unique features of this case are the ongoing fusion rhythm seen in the first Holter monitor strip (Figure, A) and the occurrence of this NSVT during pregnancy, when the choice of antiarrhythmic drugs safe for the fetus (such as flecainide) is limited.3 Fortunately, she had no hemodynamically significant arrhythmias to mandate more aggressive treatment. Expectedly, β-blockers can cause pseudofetal distress at delivery and small fetal size.
Take-home Points
With regard to differentiating VT with fusion from SVT with aberrancy, a shorter PR duration than baseline favors VT, as does atrioventricular dissociation.
The most common idiopathic ventricular arrhythmias observed in young healthy patients in the absence of structural heart disease are outflow tract ventricular arrhythmias, most commonly RVOT arrhythmias.
Management of outflow tract PVCs and VT often begins with β-blockers but can necessitate antiarrhythmic drugs or catheter ablation. The latter often has highest success.
[10]. Jacob B. Pierce, et al. Contemporary Use of Sodium-Glucose Cotransporter-2 Inhibitor Therapy Among Patients Hospitalized for Heart Failure With Reduced Ejection Fraction in the US. The Get With The GuidelinesHeart Failure Registry. JAMA Cardiol. 2023.
Question: What is the prevalence and variability of sodium-glucose cotransporter-2 inhibitor (SGLT2i) use among patients with heart failure with reduced ejection fraction (HFrEF) in the US?
Findings: In this cohort study including 49 399 patients hospitalized for HFrEF in the Get With The GuidelinesHeart Failure registry, 1 in 5 patients were discharged with prescriptions for SGLT2i therapy; rates were similarly low in patients with comorbid type 2 diabetes and chronic kidney disease. Hospital-level discharge prescription of SGLT2i varied widely, independent of patient and hospital characteristics.
Meaning: In this study, use of SGLT2i for HFrEF in the US was overall low and highly variable across hospitals.
Importance: Clinical guidelines for patients with heart failure with reduced ejection fraction (HFrEF) strongly recommend treatment with a sodium-glucose cotransporter-2 inhibitor (SGLT2i) to reduce cardiovascular mortality or HF hospitalization. Nationwide adoption of SGLT2i for HFrEF in the US is unknown.
Objective: To characterize patterns of SGLT2i use among eligible US patients hospitalized for HFrEF.
Design, Setting, and Participants: This retrospective cohort study analyzed 49 399 patients hospitalized for HFrEF across 489 sites in the Get With The GuidelinesHeart Failure (GWTG-HF) registry between July 1, 2021, and June 30, 2022. Patients with an estimated glomerular filtration rate less than 20 mL/min/1.73 m2, type 1 diabetes, and previous intolerance to SGLT2i were excluded.
Main Outcomes and Measures: Patient-level and hospital-level prescription of SGLT2i at hospital discharge.
Results: Of 49 399 included patients, 16 548 (33.5%) were female, and the median (IQR) age was 67 (56-78) years. Overall, 9988 patients (20.2%) were prescribed an SGLT2i. SGLT2i prescription was less likely among patients with chronic kidney disease (CKD; 4550 of 24 437 [18.6%] vs 5438 of 24 962 [21.8%]; P<.001) but more likely among patients with type 2 diabetes (T2D; 5721 of 21 830 [26.2%] vs 4262 of 27 545 [15.5%]; P<.001) and those with both T2D and CKD (2905 of 12 236 [23.7%] vs 7078 vs 37 139 [19.1%]; P<.001). Patients prescribed SGLT2i therapy were more likely to be prescribed background triple therapy with an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptorneprilysin inhibitor, β-blocker, and mineralocorticoid receptor antagonist (4624 of 9988 [46.3%] vs 10 880 of 39 411 [27.6%]; P<.001), and 4624 of 49 399 total study patients (9.4%) were discharged with prescriptions for quadruple medical therapy including SGLT2i. Among 461 hospitals with 10 or more eligible discharges, 19 hospitals (4.1%) discharged 50% or more of patients with prescriptions for SGLT2i, whereas 344 hospitals (74.6%) discharged less than 25% of patients with prescriptions for SGLT2i (including 29 [6.3%] that discharged zero patients with SGLT2i prescriptions). There was high between-hospital variance in the rate of SGLT2i prescription in unadjusted models (median odds ratio, 2.53; 95% CI, 2.36-2.74) and after adjustment for patient and hospital characteristics (median odds ratio, 2.51; 95% CI, 2.34-2.71).
Conclusions and Relevance: In this study, prescription of SGLT2i at hospital discharge among eligible patients with HFrEF was low, including among patients with comorbid CKD and T2D who have multiple indications for therapy, with substantial variation among US hospitals. Further efforts are needed to overcome implementation barriers and improve use of SGLT2i among patients with HFrEF.
[11]. A good read, not just for the geriatrician, but for all clinicians. BMJ 2023;381:p1013.
Lucy Pollock teaches her trainees to accept uncertainty and use all their senses. Read the signals and watch whats going on; dont just listen with your ears, listen with your whole body. Then try and focus on what matters most, she says.
Algorithmic medicine does not work in people with complex medical problems, so listen to your patient.
Pollock also tells her trainees not to avoid difficult conversations. Dont be afraid of talking about death and dyingto lots of people thats a great relief. Make sure you know what your patient wants.
Pollock, a consultant geriatrician at Somerset NHS Foundation Trust, was born into a medical familyher father was a geriatrician and her mother an ophthalmologistso she knew early on that a career in medicine would be hard work but rewarding.
My father really liked people and found great joy and humour in the dealings he had with his patients, she recalls.
Graduating from St Bartholomews Medical School in 1990, Pollock worked as a junior doctor in London before moving to Somerset, where she completed her specialist training and took up her consultant post in 2001.
Pollock has held several clinical lead and director roles and says that anyone considering medical leadership should know that life will never be quite the same again.
Its a good idea to get some training before stepping into one of these roles, and to work out who your support network is going to be, what motivates the people above you, and in what format theyre going to need information, she says. Buckle in, its hardchoose your battles, and if youre losing that battle, go and fight it a different way.
Pollock recently published The Book About Getting Older, which aims to empower patients with information. I realised a long time ago that too often I had the power in the room, and my patient and their family didnt, she explains. I felt strongly that that needed redressing. I wanted to give people that power and the information they needed in a palatable way.
She honed the skills needed to write a book through writing clinic letters. These letters can be a therapeutic device when done well, Pollock believes, and she often uses them to reassure carers that they are doing a good job. Lots of older people care for other older people and it can be a lonely job, she says. A phrase I often use is: ‘You are doing all the right things. Thats an important message.
Outside of work, Pollock loves reading, music, and spending time outdoors. She has, in her own words, lots of very funny friends and enjoys doing lots of things not very well. This, she says, helps her to have a sunny disposition. It doesnt mean Im always happy; I think the pandemic has left its mark on all of us. But optimism is a much more productive state of mind than pessimism.
[12]. Pierre-François Dequin, et al. Hydrocortisone in Severe Community-Acquired Pneumonia. N Engl J Med 2023;388:1931-1941.
Background
Whether the antiinflammatory and immunomodulatory effects of glucocorticoids may decrease mortality among patients with severe community-acquired pneumonia is unclear.
Methods
In this phase 3, multicenter, double-blind, randomized, controlled trial, we assigned adults who had been admitted to the intensive care unit (ICU) for severe community-acquired pneumonia to receive intravenous hydrocortisone (200 mg daily for either 4 or 7 days as determined by clinical improvement, followed by tapering for a total of 8 or 14 days) or to receive placebo. All the patients received standard therapy, including antibiotics and supportive care. The primary outcome was death at 28 days.
Results
A total of 800 patients had undergone randomization when the trial was stopped after the second planned interim analysis. Data from 795 patients were analyzed. By day 28, death had occurred in 25 of 400 patients (6.2%; 95% confidence interval [CI], 3.9 to 8.6) in the hydrocortisone group and in 47 of 395 patients (11.9%; 95% CI, 8.7 to 15.1) in the placebo group (absolute difference, 5.6 percentage points; 95% CI, 9.6 to 1.7; P=0.006). Among the patients who were not undergoing mechanical ventilation at baseline, endotracheal intubation was performed in 40 of 222 (18.0%) in the hydrocortisone group and in 65 of 220 (29.5%) in the placebo group (hazard ratio, 0.59; 95% CI, 0.40 to 0.86). Among the patients who were not receiving vasopressors at baseline, such therapy was initiated by day 28 in 55 of 359 (15.3%) of the hydrocortisone group and in 86 of 344 (25.0%) in the placebo group (hazard ratio, 0.59; 95% CI, 0.43 to 0.82). The frequencies of hospital-acquired infections and gastrointestinal bleeding were similar in the two groups; patients in the hydrocortisone group received higher daily doses of insulin during the first week of treatment.
Conclusions
Among patients with severe community-acquired pneumonia being treated in the ICU, those who received hydrocortisone had a lower risk of death by day 28 than those who received placebo. (Funded by the French Ministry of Health;
[13]. Floor L.F. van Baarle et al. Platelet Transfusion before CVC Placement in Patients with Thrombocytopenia. N Engl J Med 2023;388:1956-1965.
Background
Transfusion guidelines regarding platelet-count thresholds before the placement of a central venous catheter (CVC) offer conflicting recommendations because of a lack of good-quality evidence. The routine use of ultrasound guidance has decreased CVC-related bleeding complications.
Methods
In a multicenter, randomized, controlled, noninferiority trial, we randomly assigned patients with severe thrombocytopenia (platelet count, 10,000 to 50,000 per cubic millimeter) who were being treated on the hematology ward or in the intensive care unit to receive either one unit of prophylactic platelet transfusion or no platelet transfusion before ultrasound-guided CVC placement. The primary outcome was catheter-related bleeding of grade 2 to 4; a key secondary outcome was grade 3 or 4 bleeding. The noninferiority margin was an upper boundary of the 90% confidence interval of 3.5 for the relative risk.
Results
We included 373 episodes of CVC placement involving 338 patients in the per-protocol primary analysis. Catheter-related bleeding of grade 2 to 4 occurred in 9 of 188 patients (4.8%) in the transfusion group and in 22 of 185 patients (11.9%) in the no-transfusion group (relative risk, 2.45; 90% confidence interval [CI], 1.27 to 4.70). Catheter-related bleeding of grade 3 or 4 occurred in 4 of 188 patients (2.1%) in the transfusion group and in 9 of 185 patients (4.9%) in the no-transfusion group (relative risk, 2.43; 95% CI, 0.75 to 7.93). A total of 15 adverse events were observed; of these events, 13 (all grade 3 catheter-related bleeding [4 in the transfusion group and 9 in the no-transfusion group]) were categorized as serious. The net savings of withholding prophylactic platelet transfusion before CVC placement was $410 per catheter placement.
Conclusions
The withholding of prophylactic platelet transfusion before CVC placement in patients with a platelet count of 10,000 to 50,000 per cubic millimeter did not meet the predefined margin for noninferiority and resulted in more CVC-related bleeding events than prophylactic platelet transfusion.
[14]. Edward V. Loftus et al. Upadacitinib Induction and Maintenance Therapy for Crohns Disease. N Engl J Med 2023;388:1966-1980.
Background
Upadacitinib, an oral selective Janus kinase (JAK) inhibitor, is under investigation for the treatment of Crohns disease.
Methods
In two phase 3 induction trials (U-EXCEL and U-EXCEED), we randomly assigned patients with moderate-to-severe Crohns disease to receive 45 mg of upadacitinib or placebo (2:1 ratio) once daily for 12 weeks. Patients who had a clinical response to upadacitinib induction therapy were randomly assigned in the U-ENDURE maintenance trial to receive 15 mg of upadacitinib, 30 mg of upadacitinib, or placebo (1:1:1 ratio) once daily for 52 weeks. The primary end points for induction (week 12) and maintenance (week 52) were clinical remission (defined as a Crohns Disease Activity Index score of <150 [range, 0 to 600, with higher scores indicating more severe disease activity]) and endoscopic response (defined as a decrease in the Simple Endoscopic Score for Crohns Disease [SES-CD; range, 0 to 56, with higher scores indicating more severe disease] of >50% from baseline of the induction trial [or for patients with an SES-CD of 4 at baseline, a decrease of ≥2 points from baseline]).
Results
A total of 526 patients underwent randomization in U-EXCEL, 495 in U-EXCEED, and 502 in U-ENDURE. A significantly higher percentage of patients who received 45-mg upadacitinib than those who received placebo had clinical remission (in U-EXCEL, 49.5% vs. 29.1%; in U-EXCEED, 38.9% vs. 21.1%) and an endoscopic response (in U-EXCEL, 45.5% vs. 13.1%; in U-EXCEED, 34.6% vs. 3.5%) (P<0.001 for all comparisons). At week 52 in U-ENDURE, a higher percentage of patients had clinical remission with 15-mg upadacitinib (37.3%) or 30-mg upadacitinib (47.6%) than with placebo (15.1%), and a higher percentage had an endoscopic response with 15-mg upadacitinib (27.6%) or 30-mg upadacitinib (40.1%) than with placebo (7.3%) (P<0.001 for all comparisons). Herpes zoster infections occurred more frequently in the 45-mg and 30-mg upadacitinib groups than in the respective placebo groups, and hepatic disorders and neutropenia were more frequent in the 30-mg upadacitinib group than in the other maintenance groups. Gastrointestinal perforations developed in 4 patients who received 45-mg upadacitinib and in 1 patient each who received 30-mg or 15-mg upadacitinib.
Conclusions
Upadacitinib induction and maintenance treatment was superior to placebo in patients with moderate-to-severe Crohns disease.
[15]. Urs Fischer et al. Early versus Later Anticoagulation for Stroke with Atrial Fibrillation. 2023.
Background
The effect of early as compared with later initiation of direct oral anticoagulants (DOACs) in persons with atrial fibrillation who have had an acute ischemic stroke is unclear.
Methods
We performed an investigator-initiated, open-label trial at 103 sites in 15 countries. Participants were randomly assigned in a 1:1 ratio to early anticoagulation (within 48 hours after a minor or moderate stroke or on day 6 or 7 after a major stroke) or later anticoagulation (day 3 or 4 after a minor stroke, day 6 or 7 after a moderate stroke, or day 12, 13, or 14 after a major stroke). Assessors were unaware of the trial-group assignments. The primary outcome was a composite of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization. Secondary outcomes included the components of the composite primary outcome at 30 and 90 days.
Results
Of 2013 participants (37% with minor stroke, 40% with moderate stroke, and 23% with major stroke), 1006 were assigned to early anticoagulation and 1007 to later anticoagulation. A primary-outcome event occurred in 29 participants (2.9%) in the early-treatment group and 41 participants (4.1%) in the later-treatment group (risk difference, 1.18 percentage points; 95% confidence interval [CI], 2.84 to 0.47) by 30 days. Recurrent ischemic stroke occurred in 14 participants (1.4%) in the early-treatment group and 25 participants (2.5%) in the later-treatment group (odds ratio, 0.57; 95% CI, 0.29 to 1.07) by 30 days and in 18 participants (1.9%) and 30 participants (3.1%), respectively, by 90 days (odds ratio, 0.60; 95% CI, 0.33 to 1.06). Symptomatic intracranial hemorrhage occurred in 2 participants (0.2%) in both groups by 30 days.
Conclusions
In this trial, the incidence of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death at 30 days was estimated to range from 2.8 percentage points lower to 0.5 percentage points higher (based on the 95% confidence interval) with early than with later use of DOACs. (Funded by the Swiss National Science Foundation and others; ELAN ClinicalTrials..)
[16]. Lu Ma, et al. The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. 2023
Early control of elevated blood pressure is the most promising treatment for acute intracerebral haemorrhage. We aimed to establish whether implementing a goal-directed care bundle incorporating protocols for early intensive blood pressure lowering and management algorithms for hyperglycaemia, pyrexia, and abnormal anticoagulation, implemented in a hospital setting, could improve outcomes for patients with acute spontaneous intracerebral haemorrhage.
Methods
We performed a pragmatic, international, multicentre, blinded endpoint, stepped wedge cluster randomised controlled trial at hospitals in nine low-income and middle-income countries (Brazil, China, India, Mexico, Nigeria, Pakistan, Peru, Sri Lanka, and Viet Nam) and one high-income country (Chile). Hospitals were eligible if they had no or inconsistent relevant, disease-specific protocols, and were willing to implement the care bundle to consecutive patients (aged ≥18 years) with imaging-confirmed spontaneous intracerebral haemorrhage presenting within 6 h of the onset of symptoms, had a local champion, and could provide the required study data. Hospitals were centrally randomly allocated using permuted blocks to three sequences of implementation, stratified by country and the projected number of patients to be recruited over the 12 months of the study period. These sequences had four periods that dictated the order in which the hospitals were to switch from the control usual care procedure to the intervention implementation of the care bundle procedure to different clusters of patients in a stepped manner. To avoid contamination, details of the intervention, sequence, and allocation periods were concealed from sites until they had completed the usual care control periods. The care bundle protocol included the early intensive lowering of systolic blood pressure (target <140 mm Hg), strict glucose control (target 6178 mmol/L in those without diabetes and 78100 mmol/L in those with diabetes), antipyrexia treatment (target body temperature ≤375°C), and rapid reversal of warfarin-related anticoagulation (target international normalised ratio <15) within 1 h of treatment, in patients where these variables were abnormal. Analyses were performed according to a modified intention-to-treat population with available outcome data (ie, excluding sites that withdrew during the study). The primary outcome was functional recovery, measured with the modified Rankin scale (mRS; range 0 [no symptoms] to 6 [death]) at 6 months by masked research staff, analysed using proportional ordinal logistic regression to assess the distribution in scores on the mRS, with adjustments for cluster (hospital site), group assignment of cluster per period, and time (6-month periods from Dec 12, 2017). This trial is registered at Clinicaltrials.gov (NCT03209258) and the Chinese Clinical Trial Registry (ChiCTR-IOC-17011787) and is completed.
Findings
Between May 27, 2017, and July 8, 2021, 206 hospitals were assessed for eligibility, of which 144 hospitals in ten countries agreed to join and were randomly assigned in the trial, but 22 hospitals withdrew before starting to enrol patients and another hospital was withdrawn and their data on enrolled patients was deleted because regulatory approval was not obtained. Between Dec 12, 2017, and Dec 31, 2021, 10857 patients were screened but 3821 were excluded. Overall, the modified intention-to-treat population included 7036 patients enrolled at 121 hospitals, with 3221 assigned to the care bundle group and 3815 to the usual care group, with primary outcome data available in 2892 patients in the care bundle group and 3363 patients in the usual care group. The likelihood of a poor functional outcome was lower in the care bundle group (common odds ratio 086; 95% CI 076097; p=0015). The favourable shift in mRS scores in the care bundle group was generally consistent across a range of sensitivity analyses that included additional adjustments for country and patient variables (084; 073097; p=0017), and with different approaches to the use of multiple imputations for missing data. Patients in the care bundle group had fewer serious adverse events than those in the usual care group (160% vs 201%; p=00098).
Interpretation
Implementation of a care bundle protocol for intensive blood pressure lowering and other management algorithms for physiological control within several hours of the onset of symptoms resulted in improved functional outcome for patients with acute intracerebral haemorrhage. Hospitals should incorporate this approach into clinical practice as part of active management for this serious condition
[17]. Sara Mahgoub et al. Bariatricmetabolic surgery versus lifestyle intervention in non-alcoholic steatohepatitis. Lancet 2023;401(10390):P1747-1749.
Non-alcoholic fatty liver disease (NAFLD) is a growing public health issue, set to be the major cause of liver transplantation and liver cancer, with increasing socioeconomic costs.1 NAFLD is strongly associated with obesity (BMI ≥30 kg/m2) and metabolic syndrome, with an increased standardised mortality ratio of 134 compared with the general population.2 Despite progress being made regarding our understanding of the cause and pathophysiology of NAFLD, there are no approved treatments for NAFLD. Physical activity and weight-loss strategies are the mainstay for the treatment of non-alcoholic steatohepatitis (NASH), with aerobic and resistance training having been shown to reduce hepatic steatosis and NAFLD-associated cardiovascular risks.3 Bodyweight loss of more than 10% has been shown to improve fibrosis,4 but this level of weight loss is seldom reached with lifestyle change, let alone maintained in the longer term. Moreover, in an open-label study, Pais and colleagues showed that despite substantial weight loss after bariatric surgery, 47% of patients still had advanced fibrosis, although many more had improvements in fibrosis.5
In The Lancet Ornella Verrastro and colleagues6 present the first multicentre, open-label, randomised trial randomly assigning 288 patients with biopsy-proven NASH to either an intensive lifestyle intervention with best medical care (n=96 [33%]), Roux-en-Y gastric bypass (n=96 [33%]), or sleeve gastrectomy (n=96 [33%]). The study was done at three hospitals in Rome, Italy. 153 (53%) patients were male, 135 (47%) were female, all were White, and they had a BMI of 3055 kg/m2. 236 (81%) patients completed the trial. In the intention-to-treat analysis, 54 (56%) patients who had Roux-en-Y gastric bypass and 55 (57%) in the sleeve gastrectomy group met the industry-standard primary endpoint, which was histological resolution of NASH with no worsening of fibrosis at 1 year, compared with 15 (16%) patients in the lifestyle modification group (p<00001). Those who met the primary endpoint lost more bodyweight, had higher rates of diabetes remission, and better glycaemic control compared with non-responders.
In the per-protocol analysis, improvement of fibrosis score by at least one stage without worsening of NASH was higher in the surgical groups: 35 (46%) of 76 patients in the Roux-en-Y gastric bypass group and 37 (47%) of 78 patients in the sleeve gastrectomy group, compared with 22 (28%) of 80 patients in the lifestyle modification group (p=0017). Diabetes remission was also significantly higher in the surgical groups than in the lifestyle modification group (p<00001). There were no serious adverse events reported, with expected common surgical complications being managed endoscopically or medically. The 1-year mean weight loss in the study was 55% in the lifestyle modification group, with only 272% having weight loss of 10% or more. This is the first study to show that there is little incremental benefit on liver histology of weight loss of more than 20%, suggesting the role of other factors in sustaining disease activity.
This is the first randomised trial studying the effects of bariatric surgery with the conventional industry-standard primary endpoint of NASH resolution using liver histology. As in any study there are some limitations meriting consideration. First, the study was done in three Italian centres and, as all patients were White, the trial participants probably do not reflect the multiethnic diversity of people with NAFLD worldwide. Second, more effective pharmacological interventions for the management of obesity, including incretin hormone analogues such as semaglutide,7, 8 tirzepatide,9 and BI 456906,10 are being investigated for patients with NASH. In this study, mean baseline BMI in the lifestyle modification group was 412 (SD 64), which means that patients were also eligible for the higher dose of liraglutide at 30 mg rather than the 18 mg daily dosing used in this study. Thus, a potentially suboptimal lower dose of GLP-1 agonist was used in a third of the eligible cohort. Third, most patients (n=139 [48%]) enrolled had minimal (F1) fibrosis, with only 32 (11%) having advanced (F3) fibrosis. Because stages F3 and F4 fibrosis, rather than F1 and F2 fibrosis, are associated with increased rates of major adverse liver outcomes and major adverse cardiac events,11 it would be important to establish whether bariatric surgery was effective in these patients with more advanced liver disease. Not only are interventions likely to be limited to patients with advanced fibrosis, such patients might also be more refractory to therapies, hence the need to show effectiveness in this group.
In summary, although this study clearly shows the value of bariatric surgery in patients with NASH, questions remain about the effectiveness in those with advanced fibrosis. Although both surgical approaches were effective, further guidance is needed to inform which procedure should be recommended for specific patient groups. Finally, there is value in considering the potential utility of combining bariatric surgery with pharmacotherapy to enhance efficacy.
[18]. Eleanor J Samarasekera, et al. Cardiovascular disease risk assessment and reduction: summary of updated NICE guidance. BMJ 2023;381:p1028.
What you need to know
Manage cardiovascular disease (CVD) risk using an individualised approach, with lifestyle modification as the first step
For people without CVD, assess 10 year risk of a CVD event using QRISK3. This can be done as part of NHS health checks and for people likely to be at high risk based on data available in primary care health records
Tools that estimate the lifetime risk of CVD can be valuable to inform discussions and motivate lifestyle changes, especially in people who have modifiable CVD risk factors but a QRISK3 score less than 10% or age under 40
Statin therapy is highly cost effective for the primary and secondary prevention of CVD events at all levels of CVD risk, and recommended doses are atorvastatin 20 mg for primary prevention and atorvastatin 80 mg for secondary prevention
Cardiovascular disease (CVD) is the leading cause of death worldwide, with dyslipidaemia being a highly modifiable risk factor for CVD. In England, raised cholesterol affects 43% of adults and is estimated to account for 7.1% of all deaths and 3.7% of disability adjusted life years. For every 1 mmol/L of lowered low density lipoprotein cholesterol (LDL-C), CVD event risk is reduced by 22%, and thus lipid lowering therapy forms a cornerstone of CVD risk management, irrespective of baseline cholesterol levels. However, many people at significant risk of CVD do not receive lipid lowering therapies, or they receive inadequate treatment.
[19]. Michelle W.J. Heijman et al. Association of Low-Dose Colchicine With Incidence of Knee and Hip Replacements. 2023
Background:
Osteoarthritis is a major contributor to pain and disability worldwide. Given that inflammation plays an important role in the development of osteoarthritis, anti-inflammatory drugs may slow disease progression.
Objective:
To examine whether colchicine, 0.5 mg daily, reduces incident total knee replacements (TKRs) and total hip replacements (THRs).
Design:
Exploratory analysis of the LoDoCo2 (Low-Dose Colchicine 2) randomized, controlled, double-blind trial. (Australian New Zealand Clinical Trials Registry: ACTRN12614000093684)
Setting:
43 centers in Australia and the Netherlands.
Patients:
5522 patients with chronic coronary artery disease.
Intervention:
Colchicine, 0.5 mg, or placebo once daily.
Measurements:
The primary outcome was time to first TKR or THR since randomization. All analyses were performed on an intention-to-treat basis.
Results:
A total of 2762 patients received colchicine and 2760 received placebo during a median follow-up of 28.6 months. During the trial, TKR or THR was performed in 68 patients (2.5%) in the colchicine group and 97 (3.5%) in the placebo group (incidence rate, 0.90 vs. 1.30 per 100 person-years; incidence rate difference, 0.40 [95% CI, 0.74 to 0.06] per 100 person-years; hazard ratio, 0.69 [CI, 0.51 to 0.95]). In sensitivity analyses, similar results were obtained when patients with gout at baseline were excluded and when joint replacements that occurred in the first 3 and 6 months of follow-up were omitted.
Limitation:
LoDoCo2 was not designed to investigate the effect of colchicine in osteoarthritis of the knee or hip and did not collect information specifically on osteoarthritis.
Conclusion:
In this exploratory analysis of the LoDoCo2 trial, use of colchicine, 0.5 mg daily, was associated with a lower incidence of TKR and THR. Further investigation of colchicine therapy to slow disease progression in osteoarthritis is warranted.
Primary Funding Source:
None.
[20]. Miriam Santer, et al. Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial. BMJ2023;381:e074349.
Abstract
ObjectiveTo assess the effectiveness of oral spironolactone for acne vulgaris in adult women.
Design
Pragmatic, multicentre, phase 3, double blind, randomised controlled trial.
Setting
Primary and secondary healthcare, and advertising in the community and on social media in England and Wales.ParticipantsWomen (≥18 years) with facial acne for at least six months, judged to warrant oral antibiotics.
Interventions
Participants were randomly assigned (1:1) to either 50 mg/day spironolactone or matched placebo until week six, increasing to 100 mg/day spironolactone or placebo until week 24. Participants could continue using topical treatment.
Main outcome measures
Primary outcome was Acne-Specific Quality of Life (Acne-QoL) symptom subscale score at week 12 (range 0-30, where higher scores reflect improved QoL). Secondary outcomes were Acne-QoL at week 24, participant self-assessed improvement; investigators global assessment (IGA) for treatment success; and adverse reactions.
Results
From 5 June 2019 to 31 August 2021, 1267 women were assessed for eligibility, 410 were randomly assigned to the intervention (n=201) or control group (n=209) and 342 were included in the primary analysis (n=176 in the intervention group and n=166 in the control group). Baseline mean age was 29.2 years (standard deviation 7.2), 28 (7%) of 389 were from ethnicities other than white, with 46% mild, 40% moderate, and 13% severe acne. Mean Acne-QoL symptom scores at baseline were 13.2 (standard deviation 4.9) and at week 12 were 19.2 (6.1) for spironolactone and 12.9 (4.5) and 17.8 (5.6) for placebo (difference favouring spironolactone 1.27 (95% confidence interval 0.07 to 2.46), adjusted for baseline variables). Scores at week 24 were 21.2 (5.9) for spironolactone and 17.4 (5.8) for placebo (difference 3.45 (95% confidence interval 2.16 to 4.75), adjusted). More participants in the spironolactone group reported acne improvement than in the placebo group: no significant difference was reported at week 12 (72%v68%, odds ratio 1.16 (95% confidence interval 0.70 to 1.91)) but significant difference was noted at week 24 (82%v63%, 2.72 (1.50 to 4.93)). Treatment success (IGA classified) at week 12 was 31 (19%) of 168 given spironolactone and nine (6%) of 160 given placebo (5.18 (2.18 to 12.28)). Adverse reactions were slightly more common in the spironolactone group with more headaches reported (20%v12%; p=0.02). No serious adverse reactions were reported
Conclusions
Spironolactone improved outcomes compared with placebo, with greater differences at week 24 than week 12. Spironolactone is a useful alternative to oral antibiotics for women with acne.
What is already known on this TOPIC
First line treatments for acne are fixed combination topical therapies, but many people receive second line treatments, including long courses of oral antibiotics, leading to antibiotic resistance. Spironolactone is used off license by dermatologists for acne in women because of its anti-androgenic properties. Evidence for the benefit of spironolactone in acne is not robust
What this study adds
Spironolactone improved acne on all outcomes: not all outcomes were significant at 12 weeks, but all were significant at 24 weeks. Spironolactone at doses of 50 mg and 100 mg were well tolerated with mild side effects similar to placebo. Spironolactone could provide a useful alternative to oral antibiotics for women with persistent acne where first line topical treatments have not worked
[21]. Timothy S. Anderson et al. Clinical Outcomes of Intensive Inpatient Blood Pressure Management in Hospitalized Older Adults. JAMA Intern Med. 2023.
Question
What is the association between intensive treatment of elevated inpatient blood pressures and clinical outcomes of hospitalized older veterans?
Findings
In this cohort study that used propensity score overlap weighting in 66 140 older adults hospitalized for noncardiac conditions, receipt of intensive inpatient antihypertensive treatment was associated with a greater risk of adverse events, with still greater risks for patients receiving intravenous antihypertensives.
Meaning
The findings do not support the treatment of elevated inpatient blood pressures in hospitalized older adults without evidence of end organ damage and highlight the need for randomized clinical trials of inpatient blood pressure treatment targets.
Importance
Asymptomatic blood pressure (BP) elevations are common in hospitalized older adults, and widespread heterogeneity in the clinical management of elevated inpatient BPs exists.
Objective
To examine the association of intensive treatment of elevated inpatient BPs with in-hospital clinical outcomes of older adults hospitalized for noncardiac conditions.
Design, Setting, and participants
This retrospective cohort study examined Veterans Health Administration data between October 1, 2015, and December 31, 2017, for patients aged 65 years or older hospitalized for noncardiovascular diagnoses and who experienced elevated BPs in the first 48 hours of hospitalization.
Interventions
Intensive BP treatment following the first 48 hours of hospitalization, defined as receipt of intravenous antihypertensives or oral classes not used prior to admission.
Main Outcome and Measures
The primary outcome was a composite of inpatient mortality, intensive care unit transfer, stroke, acute kidney injury, B-type natriuretic peptide elevation, and troponin elevation. Data were analyzed between October 1, 2021, and January 10, 2023, with propensity score overlap weighting used to adjust for confounding between those who did and did not receive early intensive treatment.
Results
Among 66 140 included patients (mean [SD] age, 74.4 [8.1] years; 97.5% male and 2.6% female; 17.4% Black, 1.7% Hispanic, and 75.9% White), 14 084 (21.3%) received intensive BP treatment in the first 48 hours of hospitalization. Patients who received early intensive treatment vs those who did not continued to receive a greater number of additional antihypertensives during the remainder of their hospitalization (mean additional doses, 6.1 [95% CI, 5.8-6.4] vs 1.6 [95% CI, 1.5-1.8], respectively). Intensive treatment was associated with a greater risk of the primary composite outcome (1220 [8.7%] vs 3570 [6.9%]; weighted odds ratio [OR], 1.28; 95% CI, 1.18-1.39), with the highest risk among patients receiving intravenous antihypertensives (weighted OR, 1.90; 95% CI, 1.65-2.19). Intensively treated patients were more likely to experience each component of the composite outcome except for stroke and mortality. Findings were consistent across subgroups stratified by age, frailty, preadmission BP, early hospitalization BP, and cardiovascular disease history.
Conclusions and Relevance
The studys findings indicate that among hospitalized older adults with elevated BPs, intensive pharmacologic antihypertensive treatment was associated with a greater risk of adverse events. These findings do not support the treatment of elevated inpatient BPs without evidence of end organ damage, and they highlight the need for randomized clinical trials of inpatient BP treatment targets.
[22]. Sharangini Rajesh et al. Head injury: assessment and early managementsummary of updated NICE guidance. BMJ 2023;381:p1130.
What you need to know
Consider an intravenous tranexamic acid bolus within 2 hours of injury in people with suspected moderate or severe traumatic brain injury, even when no extracranial bleeding is evident
Shared decision making can inform a decision not to conduct a computed tomography head scan in people taking anticoagulant or antiplatelet medication if there are no signs or symptoms of traumatic brain injury
Any severity of head injury can cause symptoms of pituitary dysfunction. Consider investigations or referral for hypopituitarism in those with persistent symptoms consistent with hypopituitarism
Consider referring people who have persisting symptoms following a head injury to appropriate clinicians or a multidisciplinary team
More than 600000 people attend emergency departments annually in England and Wales with a recent head injury.1 High quality early management for people with head injury can prevent death from secondary brain injury. Traumatic brain injury is the major contributor to death and disability resulting from major trauma.
The National Institute for Health and Care Excellence (NICE) first published guidance on the assessment and early management of head injury in babies, children, young people, and adults in 2003 and last updated guidance in 2014.23 The key drivers for this update published in May 2023 include appraisal of new evidence concerning the role of tranexamic acid in people with head injury, the risks of bleeding after head injury in people taking anticoagulation and antiplatelet therapy, and the need to consider hypopituitarism as both an immediate and delayed complication after head injury of any severity. This guideline summary will cover selected recommendations, focusing on those most relevant to primary, pre-hospital and emergency department care.
[23]. Alexander Alexiou, et al. Alcohol withdrawal. BMJ 2023;381:p951.
What you need to know
Suspect acute or imminent alcohol withdrawal in any patient who is alcohol dependent and has stopped or reduced their alcohol intake within hours or days of presentation
Common symptoms include anxiety, nausea or vomiting, autonomic dysfunction, and insomnia. These may progress to severe withdrawal with seizures and alcohol withdrawal delirium
Alcohol withdrawal delirium is a life threatening medical emergency that requires urgent treatment with a benzodiazepine. Involve senior support and critical care
Not all patients with symptoms of alcohol withdrawal will need acute drug treatment; those with mild to moderate alcohol withdrawal symptoms can generally be managed with supportive care only
Start a benzodiazepine regimen (fixed dose or symptom triggered, depending on the clinical setting) for any patient needing acute drug treatment
Alcohol withdrawal occurs in patients who are alcohol dependent and who have stopped or reduced their alcohol intake within hours or days of presentation.
Common symptoms include anxiety, nausea or vomiting, autonomic dysfunction, and insomnia.
If alcohol withdrawal is not treated or is inadequately treated, 5% of patients will progress to alcohol withdrawal delirium (also known as delirium tremens).
Alcohol withdrawal delirium is characterised by hallucinations, delusions, profound confusion and delirium, coarse tremor, and features of clinical instability.
This is a life threatening feature of severe alcohol withdrawal and generally occurs 48 to 72 hours after the last consumption of alcohol
[24]. Wenjie Zi, M.D et al. Tirofiban for Stroke without Large or Medium-Sized Vessel Occlusion. N Engl J Med. 2023;388:2025-2036.
Background
The effects of the glycoprotein IIb/IIIa receptor inhibitor tirofiban in patients with acute ischemic stroke but who have no evidence of complete occlusion of large or medium-sized vessels have not been extensively studied.
Methods
In a multicenter trial in China, we enrolled patients with ischemic stroke without occlusion of large or medium-sized vessels and with a National Institutes of Health Stroke Scale score of 5 or more and at least one moderately to severely weak limb. Eligible patients had any of four clinical presentations: ineligible for thrombolysis or thrombectomy and within 24 hours after the patient was last known to be well; progression of stroke symptoms 24 to 96 hours after onset; early neurologic deterioration after thrombolysis; or thrombolysis with no improvement at 4 to 24 hours. Patients were assigned to receive intravenous tirofiban (plus oral placebo) or oral aspirin (100 mg per day, plus intravenous placebo) for 2 days; all patients then received oral aspirin until day 90. The primary efficacy end point was an excellent outcome, defined as a score of 0 or 1 on the modified Rankin scale (range, 0 [no symptoms] to 6 [death]) at 90 days. Secondary end points included functional independence at 90 days and a quality-of-life score. The primary safety end points were death and symptomatic intracranial hemorrhage.
Results
A total of 606 patients were assigned to the tirofiban group and 571 to the aspirin group. Most patients had small infarctions that were presumed to be atherosclerotic. The percentage of patients with a score of 0 or 1 on the modified Rankin scale at 90 days was 29.1% with tirofiban and 22.2% with aspirin (adjusted risk ratio, 1.26; 95% confidence interval, 1.04 to 1.53, P=0.02). Results for secondary end points were generally not consistent with the results of the primary analysis. Mortality was similar in the two groups. The incidence of symptomatic intracranial hemorrhage was 1.0% in the tirofiban group and 0% in the aspirin group.
Conclusions
In this trial involving heterogeneous groups of patients with stroke of recent onset or progression of stroke symptoms and nonoccluded large and medium-sized cerebral vessels, intravenous tirofiban was associated with a greater likelihood of an excellent outcome than low-dose aspirin. Incidences of intracranial hemorrhages were low but slightly higher with tirofiban.
[25]. Gregg W. Stone, et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N Engl J Med 2023;388:2037-2048.
Background
Data from a 5-year follow-up of outcomes after transcatheter edge-to-edge repair of severe mitral regurgitation, as compared with outcomes after maximal doses of guideline-directed medical therapy alone, in patients with heart failure are now available.
Methods
We randomly assigned patients with heart failure and moderate-to-severe or severe secondary mitral regurgitation who remained symptomatic despite the use of maximal doses of guideline-directed medical therapy to undergo transcatheter edge-to-edge repair plus receive medical therapy (device group) or to receive medical therapy alone (control group) at 78 sites in the United States and Canada. The primary effectiveness end point was all hospitalizations for heart failure through 2 years of follow-up. The annualized rate of all hospitalizations for heart failure, all-cause mortality, the risk of death or hospitalization for heart failure, and safety, among other outcomes, were assessed through 5 years.
Results
Of the 614 patients enrolled in the trial, 302 were assigned to the device group and 312 to the control group. The annualized rate of hospitalization for heart failure through 5 years was 33.1% per year in the device group and 57.2% per year in the control group (hazard ratio, 0.53; 95% confidence interval [CI], 0.41 to 0.68). All-cause mortality through 5 years was 57.3% in the device group and 67.2% in the control group (hazard ratio, 0.72; 95% CI, 0.58 to 0.89). Death or hospitalization for heart failure within 5 years occurred in 73.6% of the patients in the device group and in 91.5% of those in the control group (hazard ratio, 0.53; 95% CI, 0.44 to 0.64). Device-specific safety events within 5 years occurred in 4 of 293 treated patients (1.4%), with all the events occurring within 30 days after the procedure.
Conclusions
Among patients with heart failure and moderate-to-severe or severe secondary mitral regurgitation who remained symptomatic despite guideline-directed medical therapy, transcatheter edge-to-edge repair of the mitral valve was safe and led to a lower rate of hospitalization for heart failure and lower all-cause mortality through 5 years of follow-up than medical therapy alone.
[26]. Peter J. Snelling et al. Ultrasonography or Radiography for Suspected Pediatric Distal Forearm Fractures. N Engl J Med 2023;388:2049-2057.
Background
Data on whether ultrasonography for the initial diagnostic imaging of forearm fractures in children and adolescents is noninferior to radiography for subsequent physical function of the arm are limited.
Methods
In this open-label, multicenter, noninferiority, randomized trial in Australia, we recruited participants 5 to 15 years of age who presented to the emergency department with an isolated distal forearm injury, without a clinically visible deformity, in whom further evaluation with imaging was indicated. Participants were randomly assigned to initially undergo point-of-care ultrasonography or radiography, and were then followed for 8 weeks. The primary outcome was physical function of the affected arm at 4 weeks as assessed with the use of the validated Pediatric Upper Extremity Short Patient-Reported Outcomes Measurement Information System (PROMIS) score (range, 8 to 40, with higher scores indicating better function); the noninferiority margin was 5 points.
Results
A total of 270 participants were enrolled, with outcomes for 262 participants (97%) available at 4 weeks (with a window of ±3 days) as prespecified. PROMIS scores at 4 weeks in the ultrasonography group were noninferior to those in the radiography group (mean, 36.4 and 36.3 points, respectively; mean difference, 0.1 point; 95% confidence interval [CI], 1.3 to 1.4). Intention-to-treat analyses (in 266 participants with primary outcome data recorded at any time) produced similar results (mean difference, 0.1 point; 95% CI, 1.3 to 1.4). No clinically important fractures were missed, and there were no between-group differences in the occurrence of adverse events.
Conclusions
In children and adolescents with a distal forearm injury, the use of ultrasonography as the initial diagnostic imaging method was noninferior to radiography with regard to the outcome of physical function of the arm at 4 weeks. (Funded by the Emergency Medicine Foundation and others; BUCKLED Australian New Zealand Clinical Trials Registry
[27]. Nicholas C. Turner, et al. Capivasertib in Hormone ReceptorPositive Advanced Breast Cancer. N Engl J Med 2023;388:2058-2070.
Background
AKT pathway activation is implicated in endocrine-therapy resistance. Data on the efficacy and safety of the AKT inhibitor capivasertib, as an addition to fulvestrant therapy, in patients with hormone receptorpositive advanced breast cancer are limited.
Methods
In a phase 3, randomized, double-blind trial, we enrolled eligible pre-, peri-, and postmenopausal women and men with hormone receptorpositive, human epidermal growth factor receptor 2negative advanced breast cancer who had had a relapse or disease progression during or after treatment with an aromatase inhibitor, with or without previous cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor therapy. Patients were randomly assigned in a 1:1 ratio to receive capivasertib plus fulvestrant or placebo plus fulvestrant. The dual primary end point was investigator-assessed progression-free survival assessed both in the overall population and among patients with AKT pathwayaltered (PIK3CA, AKT1, or PTEN) tumors. Safety was assessed.
Results
Overall, 708 patients underwent randomization; 289 patients (40.8%) had AKT pathway alterations, and 489 (69.1%) had received a CDK4/6 inhibitor previously for advanced breast cancer. In the overall population, the median progression-free survival was 7.2 months in the capivasertibfulvestrant group, as compared with 3.6 months in the placebofulvestrant group (hazard ratio for progression or death, 0.60; 95% confidence interval [CI], 0.51 to 0.71; P<0.001). In the AKT pathwayaltered population, the median progression-free survival was 7.3 months in the capivasertibfulvestrant group, as compared with 3.1 months in the placebofulvestrant group (hazard ratio, 0.50; 95% CI, 0.38 to 0.65; P<0.001). The most frequent adverse events of grade 3 or higher in patients receiving capivasertibfulvestrant were rash (in 12.1% of patients, vs. in 0.3% of those receiving placebofulvestrant) and diarrhea (in 9.3% vs. 0.3%). Adverse events leading to discontinuation were reported in 13.0% of the patients receiving capivasertib and in 2.3% of those receiving placebo.
Conclusions
Capivasertibfulvestrant therapy resulted in significantly longer progression-free survival than treatment with fulvestrant alone among patients with hormone receptorpositive advanced breast cancer whose disease had progressed during or after previous aromatase inhibitor therapy with or without a CDK4/6 inhibitor. (Funded by AstraZeneca and the National Cancer Institute;
[28]. Enrique M. Baldessari et al. Phantom Tumor of the Lung. N Engl J Med 2023;388:2086.
A 50-year-old man with a history of mitral-valve prolapse and of Hodgkins lymphoma 20 years earlier that was treated with chemotherapy and radiation therapy presented to the emergency department with a 5-day history of dyspnea. Physical examination showed jugular venous distention, diminished breath sounds at the lung bases, and a holosystolic murmur at the cardiac apex. A radiograph of the chest showed indistinct pulmonary vascularity, a dense retrocardiac opacity, and pleural effusions on both sides. A sharply demarcated, lenticular lesion was shown in the right middle lung field (Panel A, anteriorposterior view). An echocardiogram showed an ejection fraction of 45% and severe mitral regurgitation. Treatment with diuretics and vasodilators was initiated. A follow-up chest radiograph 3 days after presentation showed complete resolution of the right lung opacity, as well as reduced pulmonary edema (Panel B, posterioranterior view). A diagnosis of phantom tumor of the lung was made. Phantom tumors of the lung, also known as vanishing tumors or pseudotumors, are loculated interlobar pleural effusions that occur most commonly in the minor fissure, as in this case. The resolution of phantom tumors with diuresis distinguishes them from true masses. Surgical coronary-artery revascularization and mitral-valve replacement were performed. At 1-month follow-up, the patient was doing well.
[29]. Prakriti Gaba, et al. Percutaneous Coronary Intervention vs Coronary Artery Bypass Graft Surgery for Left Main Disease in Patients With and Without Acute Coronary Syndromes. A Pooled Analysis of 4 Randomized Clinical Trials. JAMA Cardiol. 2023.
Question
Among patients with left main coronary artery disease, do outcomes differ for those presenting with vs without acute coronary syndrome (ACS) and by revascularization with percutaneous coronary intervention (PCI) vs coronary artery bypass graft (CABG) surgery?
Findings
In this pooled analysis of 4 randomized clinical trials, patients with ACS had more comorbidities and higher rates of early cardiovascular death over 5 years. PCI and CABG resulted in similar rates of all-cause death over 5 years regardless of ACS.
Meaning
PCI and CABG both appear to be reasonable options for revascularization in patients with left main disease presenting with or without ACS.
Importance
Patients with left main coronary artery disease presenting with an acute coronary syndrome (ACS) represent a high-risk and understudied subgroup of patients with atherosclerosis.
Objective
To assess clinical outcomes after PCI vs CABG in patients with left main disease with vs without ACS.
Design, Setting, and Participants
Data were pooled from 4 trials comparing PCI with drug-eluting stents vs CABG in patients with left main disease who were considered equally suitable candidates for either strategy (SYNTAX, PRECOMBAT, NOBLE, and EXCEL). Patients were categorized as presenting with or without ACS. Kaplan-Meier event rates through 5 years and Cox model hazard ratios were generated, and interactions were tested. Patients were enrolled in the individual trials from 2004 through 2015. Individual patient data from the trials were pooled and reconciled from 2020 to 2021, and the analyses pertaining to the ACS subgroup were performed from March 2022 through February 2023.
Main Outcomes and Measures
The primary outcome was death through 5 years. Secondary outcomes included cardiovascular death, spontaneous myocardial infarction (MI), procedural MI, stroke, and repeat revascularization.
Results
Among 4394 patients (median [IQR] age, 66 [59-73] years; 3371 [76.7%] male and 1022 [23.3%] female) randomized to receive PCI or CABG, 1466 (33%) had ACS. Patients with ACS were more likely to have diabetes, prior MI, left ventricular ejection fraction less than 50%, and higher SYNTAX scores. At 30 days, patients with ACS had higher all-cause death (hazard ratio [HR], 3.40; 95% CI, 1.81-6.37; P<.001) and cardiovascular death (HR, 3.21; 95% CI, 1.69-6.08; P<.001) compared with those without ACS. Patients with ACS also had higher rates of spontaneous MI (HR, 1.70; 95% CI, 1.25-2.31; P<.001) through 5 years. The rates of all-cause mortality through 5 years with PCI vs CABG were 10.9% vs 11.5% (HR, 0.93; 95% CI, 0.68-1.27) in patients with ACS and 11.3% vs 9.6% (HR, 1.19; 95% CI, 0.95-1.50) in patients without ACS (P=.22 for interaction). The risk of early stroke was lower with PCI vs CABG (ACS: HR, 0.39; 95% CI, 0.12-1.25; no ACS: HR, 0.35; 95% CI, 0.16-0.75), whereas the 5-year risks of spontaneous MI and repeat revascularization were higher with PCI vs CABG (spontaneous MI: ACS: HR, 1.74; 95% CI, 1.09-2.77; no ACS: HR, 3.03; 95% CI, 1.94-4.72; repeat revascularization: ACS: HR, 1.57; 95% CI, 1.19-2.09; no ACS: HR, 1.90; 95% CI, 1.54-2.33), regardless of ACS status.
Conclusion and Relevance
Among largely stable patients undergoing left main revascularization and with predominantly low to intermediate coronary anatomical complexity, those with ACS had higher rates of early death. Nonetheless, rates of all-cause mortality through 5 years were similar with PCI vs CABG in this high-risk subgroup. The relative advantages and disadvantages of PCI vs CABG in terms of early stroke and long-term spontaneous MI and repeat revascularization were consistent regardless of ACS status.
[29]. Daniel Sokol. Should doctors apologise to patients? BMJ 2023;381:p1275.
A recent decision of the medical practitioners tribunal (the tribunal service for doctors in the UK) to suspend a junior doctor for two months has led to widespread concern in the medical community. The case involves Nithya Pandian, a doctor who claims that she examined a patient when the patient denies that an examination took place.1 The tribunal had to determine whether Pandian did, in fact, examine the patient. After hearing testimony from the patient, the patients husband, and Pandian, the tribunal concluded that it was more likely than not that Pandian had not examined the patient, even though her medical notes suggest otherwise.
In a statement produced in the course of an earlier investigation by the NHS trust where Pandian worked, she had written if Patient A feels that I documented this [the abdominal examination] without examination then I sincerely apologise for all the distress that Patient A went through because of this.
In the tribunal hearing, counsel for the General Medical Council argued, without success, that Pandians apology provided evidence that she had not examined the patient. The tribunal accepted Pandians explanation that a senior colleague had advised her to apologise and held that the apology was not an admission of guilt. Yet the tribunal found the patients detailed account of the encounter with Pandian persuasive and accepted that no examination was performed.
As news of Pandians suspension broke out, the medical Twittersphere erupted in outrage over the alleged racism of the decision (Pandian obtained her medical degree in India), the apparent unfairness of the tribunals finding that no examination took place, and the GMCs reliance on the apology as evidence of wrongdoing. One doctor tweeted Perhaps in light of the MPTS and GMC findings we should stop apologising until investigations are complete. Others lamented that apologies could now be considered tantamount to an admission of guilt and called for medical defence unions to clarify if a change in practice is needed.2,3
Although the tribunal accepted that Pandian’s apology was not a confession of wrongdoing, this aspect of the case has clearly disconcerted doctors, reviving questions about whether doctors should apologise at all, the timing of apologies, and their potential impact on investigations.
When something goes wrong, it is consistent with the ethical principle of medical beneficence (doing good for the patient) and indeed common decency to say sorry. The apology should be documented in the clinical record. From my own experience as a clinical negligence barrister, I am quite certain that an early meaningful apology, along with an explanation of what happened and what will be done to avoid it happening again, would have stopped at least some clients from litigating. I say meaningful because an ill judged, insincere apology can make matters worse.
From a regulatory perspective, the General Medical Council states that doctors must be open and honest with patients if things go wrong and that they should offer an apology (Good Medical Practice, paragraph 55).2 A failure to apologise can therefore result in disciplinary action and may suggest a lack of insight on the part of the doctor.
On 1 June 2023, Pulse reported that the GMC acknowledged its error in inviting the tribunal to interpret Pandians apology as an admission of guilt.3 If correct, this is a welcome admission that should provide some reassurance to the medical profession.
In English law, a doctors apology does not, of itself, necessarily amount to an admission of negligence but the wording is key.4 A note from the operating surgeon to the patient stating Im so sorry for leaving a surgical instrument in your abdomen during the operation is persuasive evidence that an instrument was in fact left in the abdomen and hence of wrongdoing on the part of the surgical team. This could be relied on in subsequent proceedings. On the other hand, a note saying Im so sorry that you have suffered complications and the distress that these have caused you will be of limited value to lawyers in establishing breach of duty. It will, on the contrary, reflect well on the clinician.
It is no surprise that the joint guidance of the GMC and Nursing and Midwifery Council stresses that, when apologising, we do not expect you to take personal responsibility for something going wrong that was not your fault. (paragraph 16)5
NHS Resolution has produced a short booklet entitled Saying sorry, which is well worth reading. It advises using expressions such as Im sorry X happened, were truly sorry for the distress caused, or Im sorry, we have learned that….6 Doctors should not refuse to apologise for fear of adverse repercussions but they should think carefully about the wording of the apology.
Pandians case does not alter my view that, for patients and doctors, the benefits of a meaningful apology far outweigh the harms. From a practical perspective, if in doubt about whether to apologise and how to do so, doctors should seek advice from the hospital legal department or their medical defence organisation.
[30]. Monica A Tincop. Non-invasive diagnosis and monitoring of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. 2023.
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent form of chronic liver disease that poses challenges in diagnosis and risk stratification. Non-alcoholic steatohepatitis (NASH), the more progressive form of NAFLD, is particularly challenging to diagnose in the absence of histology. Liver biopsy is infrequently performed due to its invasive nature, potential for sampling error, and lack of inter-rater reliability. Non-invasive tests that can accurately identify patients with at-risk NASH (ie, individuals with biopsy-proven NASH with NAFLD activity score [NAS] ≥4 and fibrosis stage ≥2) are key tools to identify candidates for pharmacologic therapy in registrational trials for the treatment of NASH-related fibrosis. With emerging pharmacotherapy, non-invasive tests are required to track treatment response. Lastly, there is an unmet need for non-invasive tests to assess risk for clinical outcomes including progression to cirrhosis, hepatic decompensation, liver-related mortality, and overall mortality. In this Review we examine advances in non-invasive tests to diagnose and monitor NAFLD and NASH.
[31]. Chantal Mathieu et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial. Lancet 2023;401(10392):P1929-1940.
Background
Insulin icodec (icodec) is a basal insulin analogue suitable for once-weekly dosing. ONWARDS 4 aimed to assess the efficacy and safety of once-weekly icodec compared with once-daily insulin glargine U100 (glargine U100) in individuals with long-standing type 2 diabetes on a basal-bolus regimen.
Methods
In this 26-week, phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial, adults from 80 sites (outpatient clinics and hospital departments) across nine countries (Belgium, India, Italy, Japan, Mexico, the Netherlands, Romania, Russia, and the USA) with type 2 diabetes (glycated haemoglobin [HbA1c] 70100%) were randomly assigned (1:1) to receive once-weekly icodec or once-daily glargine U100 combined with 24 daily bolus insulin aspart injections. The primary outcome was change in HbA1c from baseline to week 26 (non-inferiority margin of 03 percentage points). The primary outcome was evaluated in the full analysis set (ie, all randomly assigned participants). Safety outcomes were evaluated in the safety analysis set (ie, all participants randomly assigned who received at least one dose of trial product). This trial is registered with ClinicalTrials.gov, NCT04880850.
Findings
Between May 14 and Oct 29, 2021, 746 participants were screened for eligibility, of whom 582 (78%) were randomly assigned (291 [50%] to icodec treatment and 291 [50%] to glargine U100 treatment). Participants had a mean duration of type 2 diabetes of 171 years (SD 84). At week 26, estimated mean change in HbA1c was 116 percentage points in the icodec group (baseline 829%) and 118 percentage points in the glargine U100 group (baseline 831%), showing non-inferiority for icodec versus glargine U100 (estimated treatment difference 002 percentage points [95% CI 011 to 015], p<00001). Overall, 171 (59%) of 291 participants in the icodec group and 167 (57%) of 291 participants in the glargine U100 group had an adverse event. 35 serious adverse events were reported in 22 (8%) of 291 participants in the icodec group and 33 serious adverse events were reported in 25 (9%) of 291 participants receiving glargine U100. Overall, combined level 2 and level 3 hypoglycaemia rates were similar between treatment groups. No new safety concerns were identified for icodec.
Interpretation
In people with long-standing type 2 diabetes on a basal-bolus regimen, once-weekly icodec showed similar improvements in glycaemic control, with fewer basal insulin injections, lower bolus insulin dose, and with no increase in hypoglycaemic rates compared with once-daily glargine U100. Key strengths of this trial include the use of masked continous glucose monitoring; the high trial completion rate; and the inclusion of a large, diverse, and multinational population. Limitations include the relatively short trial duration and the open-label design.
[32].On Doctoring in the COVID-19 ICU
Traci N. Adams, https://doi.org/10.7326/M23-1276, 13 June 2023
Only a few weeks into the Delta surge at our county hospital, I had descended into a state of abject hopelessness. Wave upon wave of patients with COVID-19 again flooded our hospital, most of them having declined a vaccine that might have made this particular surge preventable. Defeated and overworked, I headed toward the room of a patient whose oxygen saturation had dropped on the ventilator. The respiratory therapist had increased the fraction of inspired oxygen on the ventilator to 100%, and the patient was not recovering; her saturation hovered around 84%.
This patient was a young mother who had developed severe COVID-19 in late pregnancy and had been intubated upon delivery of the baby because of hypoxemia. As the mother of young children myself, I immediately felt especially invested in this patienta line that I had carefully tried not to cross during a pandemic in which more than 60% of the patients whom I cared for had died. Holding back emotion, I started the familiar sequence of checking ventilator mechanics, doing a bedside ultrasound to check for pneumothorax, starting inhaled nitric oxide, calling for paralytics, and getting set up to place her in a prone position.
Oxygen saturation 80%Her oxygen saturation dropped. More staff entered the room, discussing the status of her newborn and the three other children she had at home. Meanwhile, I struggled to keep up the barrier that I was so accustomed to hiding behind. Early in the pandemic, elderly patients with substantial underlying health issues made up most of our ICU population. During the Delta surge, the patients were younger and healthier, often parents of young children. The division between me as physician and them as patients felt artificial. The only thing keeping me out of a hospital bed given my work-related exposures was a vaccine series that we hoped would hold up against the latest variant.
Staring at this incredibly sick young mother brought back floods of emotion as I recalled the first few days in the hospital with my children as newborns, days that were filled with joy and utter exhaustion. However, this patient had not met her baby and was intubated and paralyzed; we were a long way from skin-to-skin time. Instead of being full of life, this patient was on the brink of death, with only our ICU staff and a number of probably inadequate interventions standing in her way.
74%As her oxygen saturation continued to fall, I felt tremendous pressure. If this patient died and left her four children as orphans, the weight of failing to save her would become unbearable. I was already so accustomed to flashbacks of difficult rooms and devastated families that I knew where I was headed. Although I would physically leave this room at the end of my shift, I would mentally be here for years. We began placing a bag valve mask on the patient, and the pace of activity in the room intensified.
Despite receiving inhaled nitric oxide and paralytics, she had only worsened. We had seen this hundreds of times and lost countless patients in this exact sequence of events. I called out orders while our weary ICU team worked in tandem to place the patient in a prone position; our minds inevitably wandered to the number of patients we had lost in this same way, except this time we were losing a mother with a baby in the neonatal ICU. I activated the ECMO team and prayed that we would have a circuit available for her.
60%Despite the prone position, paralytics, inhaled nitric oxide, and bag valve mask, the patient continued to worsen. Our nursing staff panicked. I began to cry, knowing that I had done all I could and was forced to stand here and watch her die.
50%I recognized that the only N95 I would receive for the next 4 days was wet from my tears. I pictured her children at home, wondering when Mom will get back as my own kids often do when I am gone for a long day in the ICU. Then I pictured them devastated at their mother’s funeral because I didn’t think she would ever come home.
40%Despite the best efforts of our team, we were losing her. The pressure to get her back was palpable; however, we continued to wait on the ECMO team and had exhausted our options. I placed her in a supine position again and waited. There was nothing else I could do. I am accustomed to having a fight-or-flight response in these moments of chaos; yet, this time I was frozen, watching the monitor, hoping and praying for a better outcome than that of the patients who had come before her, feeling completely helpless.
30%I knew I would be endlessly haunted by images of her children.
12%The room stood in stunned silence. We were watching her die. My best wasn’t good enough. We prepared to start compressions, and I gave epinephrine to help her plunging heart rate. For a moment, the ICU rooms that are often marked by the coordinated movements of a team that is calibrated for emergencies could only watch.
12%We stood around her bed in stunned silence. This felt like an eternity.
30%, 40%, 50%, 65%Her saturation began to improve. We had ECMO circuit available. While we placed a bag valve mask on the patient, a cannula was inserted for ECMO. She would probably survive; however, with our ECMO outcomes, it is unlikely that she will return to the life she had before. I don’t think I will either.
I was crushed by grief and anxiety throughout the pandemic as I encountered patients like this day after day for nearly 2 years. I did not know at the time whether to seek mental health support or whether my emotions were a normal response to seeing the volume and acuity of patients who overwhelmed our ICU while fearing for my safety and that of my family. I was told repeatedly to seek support if I needed it, but how could I know whether I needed help? What is a normal response to unspeakable tragedy? I would never manage my own physical health conditions, so why was I left to manage my own emotional and mental health?
Since recovering from the pandemic, I have learned that psychological distress, like COVID-19, is universal among those with substantial exposure to the virus and that only a subset of those experiencing such a significant stressor will develop diagnosable psychopathology. I have learned that I should have sought mental health support sooner because distressalthough a normative responsecan be debilitating and supportive interventions can help to lessen its impact.
The current pandemic may be over, but the next one is coming. As we prepare for the next pandemic, we should learn from our mistakes. More than 40% of frontline workers had positive results when screened for generalized anxiety disorder or major depressive disorder during the pandemic. Like I did, many of them do not know when to seek help, so we must be proactive in offering it. We must destigmatize mental health and integrate mental health support for frontline workers into future pandemic response plans and postpandemic recovery.
[33]. Adrian ODowd. Focusing on people not symptoms: the consultant psychiatrist. BMJ 2023;381:p1307.
Consultant psychiatrist Akeem Sule tells Adrian ODowd why his love of TV, film, and music helps him to inspire colleagues to treat patients more humanely.
Treating patients as human beings with a wide range of interests, and identities beyond their health problems, is perhaps what makes consultant psychiatrist Akeem Sule so popular with colleagues and those he mentors.
I will discuss fashion, books, and TV shows with patients, Sule says. Im interested in their world, and that enables you to engage on a deeper level. Im always trying to teach that to junior doctors.
He adds, I remember we had a patient who was interested in Cliff Richard. We both started singing ‘The Young Ones and the junior doctors were cringing, but that was a real connection with the patient. I was trying to tell them that your patients arent just a cluster of symptomsthey have lives and they have interests.
Sule qualified in 1997 in Nigeria before coming to work in the UK. He decided to specialise in psychiatry early on, partly inspired by his mother who was a psychiatric nurse.
Now a locum consultant psychiatrist mostly based at Essex Partnership University NHS Foundation Trust, Sule feels strongly about his work.
I like the breadth, length, and height of psychiatry, he says. Its the only specialty that makes you think about the vulnerabilities of people with mental health problems as well as thinking about their social conditions, housing, and the effects of austerity measures.
Tapping into that is where his interests and hobbies come to the fore. Ive always been interested in movies and TV shows and particularly those that focus on social determinants of health. Ive done talks1 and published2 articles, for example, on the TV show The Wire.
Everything you can think ofTV shows, music, filmcan teach you something about mental health.
In 2012, Sule, along with neuroscientist Becky Inkster, co-founded Hip Hop Psych3a social venture designed to bridge the gap between the medical community and hip hop culture.
Sule is also research associate at Wolfson College, University of Cambridge. Ive been teaching medical students in Cambridge since 2007 and you can see the excitement in their eyes when you talk about films such as Scarface and the impact of cocaine mixed with alcohol on peoples mental health. You see them coming alive.
Sule is a strong advocate for mentoring and is a member of the Association of Black Psychiatrists UK. He mentors trainees and Cambridge students, especially those from black and minority ethnic backgrounds, and keeps in contact with his former mentees to offer encouragement and advice.
Its important to do that for the next generation of doctors, he says. The NHS is at a crisis point and lots of doctors dont want to continue. He adds, With young doctors Im often struck by how they feel as if they are being swallowed up by medicine and they dont have time for other aspects of their life.
His advice is simple. Be passionate about the profession and bring all your skills, energies, and interests to it. Dont feel that, just because youre a doctor, you cant have another life. Those interests you have, they help you to understand your patient better.
Nominated by Olufemi Talabi
Dr Sule has been an incredible asset to my career. He was supportive as my clinical supervisor during my first year of core training and went out of his way to do both regular clinical and exam preparatory supervision sessions with me even with his severe clinical pressures. He ensured that my educational needs were prioritised as a trainee.
He has been very helpful with navigating training and exam challenges. He keeps in contact to offer words of encouragement and practical advice.
Ive found his work in transcultural psychiatry to be inspiring, especially around discussing popular culture and black mental health.
Olufemi Talabi is a third year core psychiatry trainee at the East of England Deanery.
[34]. Joseph Gascho. Reflections on a Posthumous Existence. JAMA Cardiol. 2023.
I got an 8 am call from the hospital: all my cultures were positive for Staphylococcus aureus. I told my wife, Honey, Im in big trouble! and indeed I was. Id had a transaortic valve replacement a year before, and as a retired cardiologist, I knew the dire implications of Staphylococcus endocarditis on a prosthetic aortic valve.
My son had taken me to the emergency department the night before with fever (temperature as high as 104 °F) and chills. It was flu season, and the physician thought I might have influenza or COVID-19 infection. But a physician friend, who knew about my prosthetic aortic valve and my fever and chills, had told my son to be sure they got blood cultures. In my fever-altered mental state, I might not have asked. My friend was so right. He also said Id be in the hospital for a month. And he was so right about that, too.
We immediately returned to the hospital where I was admittedand where I stayed for the next 40 days. I was very ill. A transesophageal echocardiogram showed that my prosthetic valve was almost completely occluded with vegetations. I was thrombocytopenic, I had septic emboli to both cerebral hemispheres, and I had a small subarachnoid bleed. I needed urgent valve replacement, but the neurologist caring for me recommended delaying surgery for weeks. However, my thoracic surgeon said we could not wait: You will die if we dont operate. You might die if we do operate, was what I heard. So they operated. They replaced my infected prosthetic valve, scraping out an abscess from my left ventricle, reconstructing my aortic root, and reimplanting my coronary arteries. My operative report said that after I came off bypass, I had a tremendous coagulopathy that lasted for 2 hours. In the ensuing weeks I had a gastrointestinal bleed, acute tubular necrosis, atrial fibrillation, and ventricular tachycardia.
But I recovered.
Now, 3 months after my surgery, my mind is drawn to a comment by the poet John Keats. Less than 2 months before his death from tuberculous, in his last extant letter, he wrote I have a habitual feeling of my real life having been past, and that I am leading a posthumous existence.1(p376)
Keats situation was different from mine. The year before he wrote that after a long ride on a cold winter day, he coughed up blood. He examined it, and trained as a physician, said, I know the colour [sic] of that blood; it is arterial blood; I cannot be deceived by that colour [sic]; that drop of blood is my death-warrant; I must die.2(p64) He knew he had consumption (tuberculosis), and tuberculosis was incurable in 1820.
Keats lives a posthumous existence through his poems. I wont live on in the minds of millions. Neither did I say, as Keats did, I must die, as my condition was not incurable. But the possibility of a catastrophe was exponentially higher than it had been the day before I became ill, and I had stared death in the face for the 7 days before surgery. These days as I work to regain function, I often think, I could be dead. I could be posthumous.
And then I wonder, what am I to do with this posthumous life that I have been granted? Obviously, remain grateful for every day, every hour Im alive. But beyond that? Get the Porsche Ive always dreamed of? Buy the condominium at the beach with an ocean view? Or, more seriously, supervise medical students at the local free clinic? Oversee a problem-based learning class at the medical school at which I worked for more than 30 years? Volunteer at the local hospice and sit with dying patients?
Some of my friends tell me that I have been spared. Sparing suggests to me that some sentient force paid special attention to me. Im not sure. In fact, Im not sure Id want to be associated with such a force anywaynot one that would spare me but not the 3-year-old with a brain tumor or the young father of 3 with metastatic colon cancer. Yes, I have been spared but by the expertise of the physicians and nurses caring for me. And by the luck of the draw.
No matter what sparing means, I wake every morning, ever so grateful to be alive. And its different from 6 months ago. Rationally or not, I feel that I have been granted additional time. But then I think: how many other potentially deadly events have I dodged unaware? What about the patch of black ice on the curve of Route 23 that I missed last night driving home at 65 mph? What about the cancer cells that perhaps arose in my pancreas when I was 50 years old, cells that were destroyed by neutrophils before they could multiply? And on that flight to Los Angeles last yearthe flock of starlings that missed the jet intake by a footmaybe the pilots were aware, but none of the passengers had a clue. All are sparings of which I am not aware, real sparings for which I dont even know to be grateful, sparings that do not cause me to examine my lifethese make me wonder what to do now.
We all lead a kind of posthumous existence. Maybe we all would do well to ponder the words of another poet, Mary Oliver, who wrote in The Summer Day, What is it you plan to do with your one wild and precious life?
[35]. Bruce S Stambler et al. Self-administered intranasal etripamil using a symptom-prompted, repeat-dose regimen for atrioventricular-nodal-dependent supraventricular tachycardia (RAPID). 2023.
Background
Etripamil is a fast-acting, intranasally administered calcium-channel blocker in development for on-demand therapy outside a health-care setting for paroxysmal supraventricular tachycardia. We aimed to evaluate the efficacy and safety of etripamil 70 mg nasal spray using a symptom-prompted, repeat-dose regimen for acute conversion of atrioventricular-nodal-dependent paroxysmal supraventricular tachycardia to sinus rhythm within 30 min.
Methods
RAPID was a multicentre, randomised, placebo-controlled, event-driven trial, conducted at 160 sites in North America and Europe as part 2 of the NODE-301 study. Eligible patients were aged at least 18 years and had a history of paroxysmal supraventricular tachycardia with sustained, symptomatic episodes (≥20 min) as documented by electrocardiogram. Patients were administered two test doses of intranasal etripamil (each 70 mg, 10 min apart) during sinus rhythm; those who tolerated the test doses were randomly assigned (1:1) using an interactive response technology system to receive either etripamil or placebo. Prompted by symptoms of paroxysmal supraventricular tachycardia, patients self-administered a first dose of intranasal 70 mg etripamil or placebo and, if symptoms persisted beyond 10 min, a repeat dose. Continuously recorded electrocardiographic data were adjudicated, by individuals masked to patient assignment, for the primary endpoint of time to conversion of paroxysmal supraventricular tachycardia to sinus rhythm for at least 30 s within 30 min after the first dose, which was measured in all patients who administered blinded study drug for a confirmed atrioventricular-nodal-dependent event. Safety outcomes were assessed in all patients who self-administered blinded study drug for an episode of perceived paroxysmal supraventricular tachycardia. This trial is registered at ClinicalTrials.gov, NCT03464019, and is complete.
Findings
Between Oct 13, 2020, and July 20, 2022, among 692 patients randomly assigned, 184 (99 from the etripamil group and 85 from the placebo group) self-administered study drug for atrioventricular-nodal-dependent paroxysmal supraventricular tachycardia, with diagnosis and timing confirmed. Kaplan-Meier estimates of conversion rates by 30 min were 64% (63/99) with etripamil and 31% (26/85) with placebo (hazard ratio 262; 95% CI 166415; p<00001). Median time to conversion was 172 min (95% CI 134265) with the etripamil regimen versus 535 min (387873) with placebo. Prespecified sensitivity analyses of the primary assessment were conducted to test robustness, yielding supporting results. Treatment-emergent adverse events occurred in 68 (50%) of 99 patients treated with etripamil and 12 (11%) of 85 patients in the placebo group, most of which were located at the administration site and were mild or moderate, and all of which were transient and resolved without intervention. Adverse events occurring in at least 5% of patients treated with etripamil were nasal discomfort (23%), nasal congestion (13%), and rhinorrhea (9%). No serious etripamil-related adverse events or deaths were reported.
Interpretation
Using a symptom-prompted, self-administered, initial and optional-repeat-dosing regimen, intranasal etripamil was well tolerated, safe, and superior to placebo for the rapid conversion of atrioventricular-nodal-dependent paroxysmal supraventricular tachycardia to sinus rhythm. This approach could empower patients to treat paroxysmal supraventricular tachycardia themselves outside of a health-care setting, and has the potential to reduce the need for additional medical interventions, such as intravenous medications given in an acute-care setting.
[36]. Miles Finbar Kiernan et al. Slowly progressive descending facial nerve paralysis indicates hereditary gelsolin amyloidosis. Lancet 2023;401(10393):P2072.
A 63-year-old woman attended our oculoplastic clinic with a 10-year history of progressive weakness of the muscles of her forehead, and drooping eyebrows and eyelids that were affecting her field of vision
Notably both her mother and brother had similar symptoms. The patient had no other significant medical history.
On examination, she was generally well; she had bilateral brow ptosis, bilateral dermatochalasis, and cutis laxa. The patient had marked weakness of the muscles supplied by the temporal branches of the facial nerve and, to a lesser extent, weakness of those supplied by the zygomatic branches (figure; video). The remaining branches of the facial nerve were relatively spared. Sensation supplied by the ophthalmic branch of the trigeminal nerve was reduced, with sparing of the maxillary and mandibular branches (figure; video). She had no upper eyelid weakness, no anisocoria, and normal extraocular movements. Mild bilateral type 2 corneal lattice dystrophy was seen on slit lamp biomicroscopy (figure, appendix) and corneal sensation was reduced. The patient had good corrected visual acuities of 6/9 in the right eye and 6/6 in the left eye. We noted no lagophahalmos but a moderate Bell’s phenomenon.
The patient’s clinical signs and family history were consistent with a diagnosis of hereditary gelsolin amyloidosis (AGel), and previous genetic testing had confirmed a known amyloidogenic mutation in the GSN gene: c.640G>A; p.(Asp214Asn).
A conservative bilateral direct brow lift and upper eyelid blepharoplasty was done with a marked improvement in the patient’s symptoms. At follow-up 1 year later, the patient remained happy with the outcome of the surgery.
Amyloidoses are a group of diseases characterised by the deposition of insoluble proteins which aggregate into distinctive fibrillar forms. Hereditary amyloid polyneuropathies are autosomal dominant in their inheritance and are defined according to the precursor protein of amyloidtransthyretin, apolipoprotein A-1, or gelsolin. AGel was first described in Finland where there are approximately 1000 carriers. The most common manifestations are ophthalmological, with lattice dystrophy; dermatological, with cutis laxa; and neurological, with cranial and peripheral neuropathies; renal amyloid deposits are also common and there may occasionally be cardiac amyloid infiltration. Facial nerve involvement has a unique presentationit is progressive, bilateral, and starts with the temporal branches, descending to the other branches of the facial nerves over time. Severity ranges from mild involutional changes to complete bilateral facial paralysis. Patients should be counselled appropriately regarding oculoplastic facial surgery. Improved visual function must be balanced against progressive facial and trigeminal neuropathy, and ultimately corneal protection in the long term.
[37]. Natalie Staplin et al. Relationship between clinic and ambulatory blood pressure and mortality: an observational cohort study in 59124 patients. Lancet 2023;401(10393):P2041-2050.
Background
Ambulatory blood pressure provides a more comprehensive assessment than clinic blood pressure, and has been reported to better predict health outcomes than clinic or home pressure. We aimed to examine associations of clinic and 24-h ambulatory blood pressure with all-cause and cardiovascular mortality in a large cohort of primary care patients referred for assessment of hypertension.
Methods
We did an observational cohort study using clinic and ambulatory blood pressure data obtained from March 1, 2004, to Dec 31, 2014, from the Spanish Ambulatory Blood Pressure Registry. This registry included patients from 223 primary care centres from the Spanish National Health System in all 17 regions of Spain. Mortality data (date and cause) were ascertained by a computerised search of the vital registry of the Spanish National Institute of Statistics. Complete data were available for age, sex, all blood pressure measures, and BMI. For each study participant, follow-up was from the date of their recruitment to the date of death or Dec 31, 2019, whichever occurred first. Cox models were used to estimate associations between usual clinic or ambulatory blood pressure and mortality, adjusted for confounders and additionally for alternative measures of blood pressure. For each measure of blood pressure, we created five groups (ie, fifths) defined by quintiles of that measure among those who subsequently died.
Findings
During a median follow-up of 97 years, 7174 (121%) of 59124 patients died, including 2361 (40%) from cardiovascular causes. J-shaped associations were observed for several blood pressure measures. Among the top four baseline-defined fifths, 24-h systolic blood pressure was more strongly associated with all-cause death (hazard ratio [HR] 141 per 1 SD increment [95% CI 136147]) than clinic systolic blood pressure (118 [113123]). After adjustment for clinic blood pressure, 24-h blood pressure remained strongly associated with all-cause deaths (HR 143 [95% CI 137149]), but the association between clinic blood pressure and all-cause death was attenuated when adjusted for 24-h blood pressure (104 [100109]). Compared with the informativeness of clinic systolic blood pressure (100%), night-time systolic blood pressure was most informative about risk of all-cause death (591%) and cardiovascular death (604%). Relative to blood pressure within the normal range, elevated all-cause mortality risks were observed for masked hypertension (HR 124 [95% CI 112137]) and sustained hypertension (124 [115132]), but not white-coat hypertension, and elevated cardiovascular mortality risks were observed for masked hypertension (137 [115163]) and sustained hypertension (138 [122155]), but not white-coat hypertension.
Interpretation
Ambulatory blood pressure, particularly night-time blood pressure, was more informative about the risk of all-cause death and cardiovascular death than clinic blood pressure
[38]. Orlando Guntinas-Lichius. Tonsillectomy in adultsto do or not to do. Lancet 2023;401(10393):P2015-2017.
Recurrent acute tonsillitis describes the repeated occurrence of acute bacterial infections of palatine tonsils with symptom-free intervals. Acute tonsillitis accounts for around a third of respiratory tract infections in primary care among people of all ages,1 with an estimated 600 million symptomatic cases of group A streptococcus tonsillitis worldwide annually.2 Recurrent episodes occur in 12% of patients with tonsillitisie, around 12000 per 100000 people.3 Beyond the relation to group A streptococcus infections, little is known about risk factors for the development of recurrent acute tonsillitis.4 Recurrent acute tonsillitis could be a genetic immunosusceptibility disease because patients younger than 12 years show some subclinical antibody deficiency and aberrant T-cell function5 and many undergo tonsillectomy. More than 30000 tonsillectomies are performed annually in the UK.6 Tonsillectomy can produce severe postoperative pain, complicated by postoperative bleeding which can result in death in rare cases. Clearly, we need evidence-based policies to guide health-care professionals for the indication of tonsillectomy.7 Unfortunately, not much has changed in the past 40 years. National guidelines are still based on the Paradise criteria from 1984,8 stating that a tonsillectomy is indicated when around seven or more treated tonsillitis episodes occur in 1 year. The Paradise criteria were based on paediatrics clinical trials and later extrapolated for adult guidelines without any justifying research in the adult population. Thus, the 2014 Cochrane Review concluded that the effectiveness of tonsillectomy is low.9 Until now, there was no basis to make confident decisions using national guidelines.7
Reflecting on this complex issue is important to understand how challenging designing this first randomised NATTINA trial was and what a milestone Janet Wilson and colleagues10 have achieved and report in The Lancet. The trial enrolled 453 adults aged 16 years or older (median age 23 years [IQR 1930]) with recurrent acute tonsillitis who met the UK tonsillectomy guideline criteria. 407 (90%) participants were White and 355 (78%) were female. 233 patients were randomly assigned to immediate tonsillectomy (of whom 172 [74%] received a tonsillectomy) and 220 to conservative management (of whom 124 [56%] adhered to treatment of self-analgesia plus antibiotics prescribed in the community). The primary outcome was the number of sore throat days collected during 24 months, which was lower in the immediate tonsillectomy group than in the conservative management group (median 23 days [IQR 1146] vs 30 days [1465]). There were fewer total sore throat days during 24 months in the immediate tonsillectomy group (intention-to-treat; incident rate 053 [95% CI 043065; p<00001]), which was also related to better quality of life scores. 37 (16%) participants had postoperative bleeding requiring readmission to hospital, all of whom received conservative management. This proportion is higher than expected but is most likely related to the study design. Overall, tonsillectomy was more cost-effective (mean difference 012 quality-adjusted life-years [009014]) than conservative management, at least in the UK health-care system. NATTINA also shows how challenging randomisation is to surgery versus conservative management. 4165 participants had to be screened to include 453 (11%) and many declined to participate. Those with more severe disease were probably unwilling to risk not receiving an operation (74 [34%] from the conservative management group requested to cross over to immediate tonsillectomy). Regardless of these limitations, NATTINA clearly shows that tonsillectomy in adults is clinically significant at reducing sore throat days and should be recommended when the patient fulfils current guideline criteria.
In the UK, and many other countries, a substantial proportion of patients undergo tonsillectomy despite not fulfilling the rather strict guideline criteria (also used in NATTINA for inclusion). However, whether tonsil surgery is also effective in less severe cases remains unclear. The other important question is whether a tonsillectomy needs to be performed or would a partial tonsillectomy or tonsillotomy (with less postoperative pain and morbidity) be sufficient? The answer will be provided by the ongoing randomised TOTO trial11 analysing whether tonsillotomy is not inferior to tonsillectomy in terms of total sore throat days during 24 months. TOTO uses the same outcome variables as NATTINA and would allow a pooled analysis of data from both trials.
At least 10% of patients with recurrent acute tonsillitis are younger than 16 years and would not have been included in NATTINA. The effect of tonsillectomy might be similar in children when the guideline criteria are fulfilled, but this hypothesis was not tested. A fundamental problem remains that using tonsillitis episodes per year as an inclusion criterion is vague and just an estimate given by the patient with substantial variability in accuracy. To date, there is no objective measure to classify the degree of pathological tonsillar inflammation.12
The long-term effects of tonsillectomy (ie, the removal of a secondary immune organ) remain controversial. Larger population-based association studies give weak hints that there might be a higher overall risk of developing cancer after a tonsillectomy,13 but long-term trials are needed. Concerning postoperative pain and the rare but severe risks, tonsillectomy is a major surgery and should become a main topic of research in otorhinolaryngology, head, and neck surgery. Based on NATTINA results, tonsillectomy should be offered to adults with severe recurrent acute tonsillitis instead of a repeated attempt of conservative therapy.
[39]. Courtney D DiNardo, et al. Acute myeloid leukaemia. Lancet 2023;401(10393):P2073-2086.
Summary
Progress in acute myeloid leukaemia treatment is occurring at an unprecedented pace. The past decade has witnessed an increasingly improved scientific understanding of the underlying biology of acute myeloid leukaemia, leading to enhanced prognostication tools and refined risk assessments, and most especially incorporating measurable residual disease (MRD) into longitudinal risk assessments. The classification of acute myeloid leukaemia has recently been updated by WHO and the International Consensus Classification (ICC). Recommendations for prognostic stratification, response assessment, and MRD determination have also been updated by the European LeukemiaNet. Treatment options have evolved substantially in the last 5 years for patients with newly diagnosed acute myeloid leukaemia, leading to improved outcomes in intensively treated patients and those more appropriate for non-intensive chemotherapy. More effective targeted treatment options in the relapsed setting are also available, further advancing the treatment armamentarium and improving patient outcomes.
[40]. Michael Bretthauer. What is my risk, doctor? How to convey disease risk and treatment effects. BMJ 2023;381:e075289.
What you need to know
Relative effects of treatments are often described in patient encounters, scientific journals, and mass media, although used alone to guide decision making they are insufficient and potentially misleading
Absolute treatment effects together with the absolute risk of disease one wants to prevent or treat are more informative and should be used instead
Discussions about action thresholds for absolute disease risk and absolute treatment effects are important in patient encounters and elsewhere in the healthcare system
Ms Olsen is a 65 year old woman with hypercholesterolaemia and hypertension. Her doctor tells her that she can reduce her risk of getting a major cardiovascular event by up to 50% if she takes a statin. Thats great, she thinks, 50% reduction is a lot! She feels happy and well informed and plans to take the statin.
After she comes home, she remembers her recent conversation with a car dealer (she really needed a new car). He told her a car he had in his lot was reduced in price by 15%. May be a good deal, she thought, and asked for the price of the car. Unfortunately it was far too high, even with the 15% off. She thought the dealer had not been honest since he did not tell her the price upfront, only the discount.
Risk for disease and for treatment effects is conveyed in many ways: relative or absolute, in percentages, hazards, or odds ratios. Some are more informative than others, and many of the most frequently used are hard to understand. This article outlines how to convey benefits, harms, and burden of interventions to patients and society in an informative way,

