Journal scan: A review of 30 recent papers of immediate clinical significance, harvested from major international journals
From the desk of the Editor-in-Chief
(1). Josh Serchen, et al. Preparing for Future Pandemics and Public Health Emergencies: An American College of Physicians Policy Position Paper. 2023
The onset of the COVID-19 pandemic revealed significant gaps in the United States pandemic and public health emergency response system. At the federal level, government responses were undercut by a lack of centralized coordination, inadequately defined responsibilities, and an under-resourced national stockpile. Contradictory and unclear guidance throughout the early months of the pandemic, along with inconsistent funding to public health agencies, also created notable variance in state and local responses. The lack of a coordinated response added pressure to an already overwhelmed health care system, which was forced to resort to rationing care and personal protective equipment, creating moral distress and trauma for health care workers and their patients. Despite these severe shortcomings, the COVID-19 pandemic also highlighted successful policies and approaches, such as Operation Warp Speed, which led to the fastest development and distribution of a vaccine in history. In this position paper, the American College of Physicians (ACP) offers several policy recommendations for enhancing federal, state, and local preparedness for future pandemic and public health emergencies. This policy paper builds on various statements produced by ACP throughout the COVID-19 pandemic, including on the ethical distribution of vaccinations and resources, conditions to resume economic and social activity, and efforts to protect the health and well-being of medical professionals, among others.
(2). Ashish K. Jha. Preparing for the Next Pandemic. 2023
Although SARS-CoV-2 infections continue to occur, the United States has exited the emergency phase of the pandemic. COVID-19 is manageable without major disruptions to the health care system and society. Effective COVID-19 vaccines and treatments are accessible to nearly all Americans (13). The current moment of recovery is an important time to reflect on what went right and what went wrong in this historic pandemic to learn how to do better next time. In this issue, the American College of Physicians (ACP) presents thoughtful recommendations of how we might do so (4).
When we first became aware of SARS-CoV-2 in early 2020, our nation was clearly not adequately prepared for what was to come. This lack of preparedness has been decades in the making: The challenges ranged from poor funding of public health and inadequate crisis coordinating mechanisms to inadequate attention paid to critical supply chains and an information ecosystem that would require a novel approach to communicating with the public (5, 6).
The ACP recommendations begin to meaningfully address those gaps, including the need for investments in public health, data infrastructure, effective communication strategies, and personnel. The Biden administration has begun this investment with substantial funds flowing to public health agencies, including the Centers for Disease Control and Prevention and local public health departments, to begin to fortify personnel and modernize data. Translating these resources into effective action will be critical.
The strengthening of supply chains is another area of substantial progress. When President Biden came into office, the key parts of the supply chain for vaccines, personal protective equipment, and other essential equipment were weak. Through both investments and sustained focus, those have been largely repaired. However, clear challenges remain in establishing a robust supply chain for all essential medical and health-related products that can withstand shocks like a global pandemic. The shortages of essential medicines that plague the United States and other nations of the world are evidence of these persisting challenges.
Although it is essential to recognize how our nation was ill prepared, it is also essential to recognize that which went very right. The position paper does thisidentifying vaccine development and telehealth as areas of success. I want to note 3 areas where we saw some success and need to redouble our efforts: building on real innovations of the pandemic, addressing the information crisis, and strengthening integration of health care and public health.
Sustaining and Scaling Pandemic Innovations
As the ACP notes, the pandemic brought groundbreaking innovations. While telehealth is widely touted, and rightly so, a closely related innovation that received less attention is the test-to-treat& programs that improved access to care in a range of communities and addressed issues of equity (7). For example, in New York City, mobile vans traveled to communities to provide people who had COVID-19 symptoms with rapid testing and treatment on the spot (8). Delivery innovations need to be scaled up beyond the context of COVID-19 as a model of care delivery for acute diseases where a trip to the physicians office or emergency department is neither needed nor feasible.
Another notable pandemic innovation was Operation Warp Speed. Partnership and collaboration between the federal government, private sector, and academia were the key ingredients of this program which delivered highly effective, safe vaccines in record time. We should model this program to develop other vaccines and treatments where progress is too slow. The Biden Administration recently launched Project Next Gen to accelerate development of the next generation of vaccines and treatments for COVID-19 with the hope of creating a sustainable model for the development of vaccines and treatments against future pandemics (9).
The Role of Physicians in the Response to the Health Information Crisis
Unfortunately, we practice medicine and public health in a polluted information environment. The pandemic has shown that an overabundance of bad information contributes to negative health outcomes (10). A top-down, central communications approach is ineffective in todays networked information ecosystem. The time for one trusted voice to speak to 330 million Americans is over. Physicians and other health care professionals are trusted sources of information for millions of Americans. They must know how to evaluate evidence and engage diverse people in well-informed conversations about health. It will be critical for organizations like ACP to take a leadership role in training physicians to more effectively share good information. This does not require physicians to go on television or social media; Those who prefer offline engagement can share good information at town halls, school board meetings, and elsewhere in the communities they serve.
Strengthening the Integration of Public Health and Health Care
Even the best-funded public health system cannot respond to a major health crisis on its own. The U.S. health care delivery system had to step up to meet needs during this pandemic that public health could not adequately address; however, moving forward, we need to invest in building the partnership between public health and health care. What might this look like? It starts with greater data integrationhealth care data and public health data rarely communicate with each other. Breaking down these silos will be critical, as will breaking down the cultural divides that separate the health care and public health communities. The ACP identifies health care workers as a reserve force for public health crises. Beginning to use that health care workforce to address the public health crises that exist todaysuch as the mental health crisis and the opioid crisiscan begin to build the partnerships that will be essential the next time a pandemic hits.
As we come out of the emergency phase of the COVID-19 pandemic, it is essential that we use the lessons learned to be well prepared for the next pandemic or other public health crises. If we do not, we risk sliding back to where things were in 2019. The choice is both stark and consequential. The ACP position paper offers thoughtful steps we must take to build on innovations and address our gaps in healthcare delivery and equity. We have made real progress on pandemic preparedness over the past 2 years. Now is the time to not just continue that work but accelerate itto ensure that when the next health crisis hits, we are ready.
(3). Helena Mertes et al. Tecovirimat Resistance in an Immunocompromised Patient With Mpox and Prolonged Viral Shedding. 2023
Tecovirimat, an inhibitor of the viral VP37 envelope-wrapping protein used to treat severe monkeypox virus (MPXV) infection, has a low barrier to resistance (1, 2). A tecovirimat-resistant MPXV variant was recently identified at the autopsy of an immunocompromised patient with mpox after prolonged tecovirimat treatment (3).
To describe the rapid selection of a tecovirimat-resistant MPXV variant during treatment of a severely immunocompromised patient with prolonged MPXV infection.
A 53-year-old man who had been vaccinated for smallpox in childhood presented in November 2022 with weight loss; a longstanding large, painful anal ulcer; and proctitis, but without skin lesions. Work-up revealed HIV-1 infection with pronounced immunosuppression (plasma viral load, 523 000 copies/mL; CD4+ T-lymphocyte count, 0.02 × 109 cells/L), chronic hepatitis B virus infection, latent syphilis, Cryptococcus neoformans antigenemia, anal Chlamydia trachomatis infection, and cytomegalovirus infection.
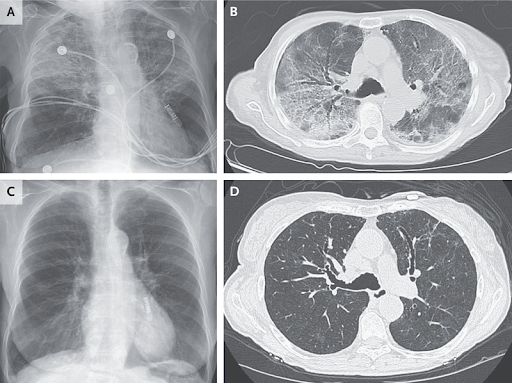
The initiation of antiretroviral therapy (ART) was deferred due to travel. Six weeks after the HIV diagnosis, the patient was admitted for worsening anal pain. Antiretroviral therapy (bictegravir, 50 mg; emtricitabine, 200 mg; and tenofovir alafenamide, 25 mg) and intravenous ganciclovir for suspected cytomegalovirus colitis were started. Perianal disease deteriorated, and necrotizing vesicles appeared on the neck and buttocks on days 15 and 20 of ART, respectively (in addition to cerebral toxoplasmosis). Mpox immune reconstitution inflammatory syndrome was suspected, and polymerase chain reaction testing of the different lesions, stored plasma samples, and anal biopsy specimens was performed. All tested positive for MPXV (Figure 1).
Figure 1. Clinical and virologic evolution over time relative to the initiation of the 2 consecutive courses of tecovirimat.
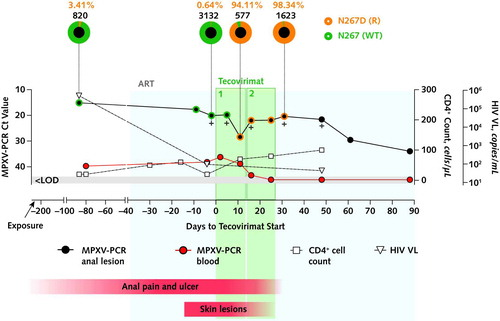
The 2 courses of tecovirimat are indicated as 1& and 2&. Sequencing results (method as described in reference 5, using an R10.4.1 flow cell [Oxford Nanopore Technologies]) are represented as pie charts above the graph: The external ring of each pie represents the proportions of wild-type (N267 [WT]; green) and resistant (N267D [R]; orange) MPXV variant populations in anal lesion samples. Numbers above each pie indicate the percentage of the N267D (R) allele frequency (orange) and the total (N267D [R] and N267 [WT]) allele depth (black) (only considering bases with a minimum quality score [Phred score] of Q30). The evolution of Ct values of anal lesions (black circles) and blood samples (red circles), HIV VL (triangles), and CD4+ cell count (squares) are presented on the graph. Crosses indicate positive MPXV culture samples. The timing of the appearance and resolution of the anal ulcer and skin lesions is indicated by the red bars underneath the graph. To convert CD4+ cell counts to ×109/L, multiply by 0.001. ART = antiretroviral therapy; Ct = PCR cycle threshold; LOD = limit of detection; MPXV = monkeypox virus; PCR = polymerase chain reaction; R = resistant; VL = viral load; WT = wild-type. Consensus sequence National Center for Biotechnology Information GenBank accession numbers: d-87 02 Nov_2022_anal: OQ672570; d-9 19_Jan_2023_anal: OQ672571; d-2 26_Jan_2023_anal: OQ672572; d-2 26_Jan_2023_neck: OQ672573; d5 02_Feb_2023_anal: OQ672574; d5 02_Feb_2023_gluteal: OQ672575; d11 08_Feb_2023_anal: OQ672576; d16 13_Feb_2023_anal: OQ672577; d25 23_Feb_2023_anal: OQ672578; d31 28_Feb_2023_anal: OQ672579.
Oral tecovirimat (600 mg twice daily) was started 1 day after the confirmation of MPXV infection (ART day 39). During the initial 2-week course, all lesions improved but anal lesion resolution was incomplete and viral shedding persisted. Tecovirimat treatment was extended for 2 weeks, after which the clinical evolution continued to be favorable. Daily intake was reliably reported throughout treatment. After 25 days of tecovirimat treatment, MPXV DNA became undetectable in blood. In contrast, except for a temporary decrease on day 11 of treatment (cycle threshold [Ct], 28.39), the anorectal viral load remained high up to day 48 (Ct, 21.58) and detectable up to the end of follow-up (Ct, 33.94 at day 88).
Retrospective MPXV sequencing of the anorectal samples revealed a dominant variant population (at 94.11% allele frequency) carrying an F13L gene mutation (encoding an N267D variant of VP37) as early as 11 days after initiation of tecovirimat treatment (Figure 1). This variant was already detectable as a minor population before tecovirimat initiation in anorectal (3.41% at day 87 and 0.64% at day 2), neck (0.23% at day 2), and gluteal (0.05% at day 11) lesion samples. In vitro, this N267D mutation was associated with a 350-fold increase in the half maximal effective concentration of tecovirimat compared with wild-type (WT) virus (2103 nM for N267D vs. 5.9 nM for WT MPXV) (Figure 2) and with reduced viral outgrowth in a direct competition assay.
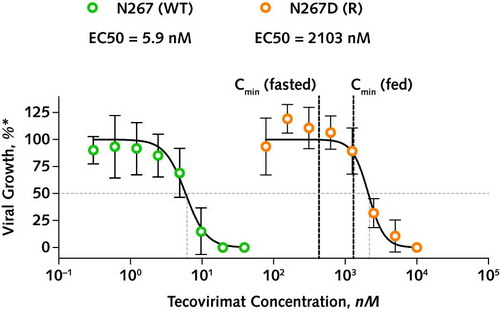
Figure 2. In vitro drug susceptibility testing.
MPXV was isolated from anal swab samples on days 2 and 31 by spinoculation on vero cells, and virus stock was prepared by double passaging on vero cells (for 3 and 4 days, respectively). Viral stocks were confirmed for the presence of WT and N267D mutant virus by whole-genome sequencing (i.e., 100% WT/0% R and 36% WT/64% R in viral stocks derived from day 2 and 31 samples; indicative of a comparative outgrowth disadvantage for the N267D mutant virus present at 98.34% in the patient sample at day 31). Serial dilutions of tecovirimat were incubated with 10 plaque-forming units (0.0005 multiplicity of infection) of dominant WT (green circles) and dominant N267D mutant virus (orange circles) containing viral stocks, on confluent vero cells for 4 days in 96 well plates in a plaque reduction assay. EC50 values were calculated using a 4-variable logistic model in GraphPad Prism v.9. Cmin = reported minimal concentration of tecovirimat (after 600-mg oral dose; from reference 1); EC50 = half maximal effective concentration; MPXV = monkeypox virus; R = resistant; WT = wild-type.
* The proportion of viral growth (expressed in amount of plaques) compared with the untreated condition.
Our report confirms the potential rapid selection of resistant mutant virus during tecovirimat monotherapy, and we believe this report is the first to study this phenomenon longitudinally. A variant (VP37 N267D) with substantiated tecovirimat resistance was selected within the standard 2-week treatment.
Although MPXV infections are mostly self-limiting, prolonged disease has been described in immunocompromised patients (4). Our patient had protracted viral shedding of at least 87 days before initiation of tecovirimat treatment, when a minor fraction of the resistant variant was already detectable. Indeed, intrahost viral evolution enhanced by prolonged replication may influence the viral populations diversity and subsequent ability to escape antiviral pressure. However, in the absence of pharmacokinetic and pharmacodynamic monitoring, we cannot rule out subtherapeutic tecovirimat levels due to factors such as insufficient high-fat food intake.
Despite the emergence of tecovirimat resistance in our patient, the clinical evolution was favorable, possibly due to further immune reconstitution. Similar to other VP37 amino acid substitutions that confer resistance to tecovirimat in orthopox viruses (2), decreased viral fitness may play a role, as suggested by the in vitro outgrowth assay. Whether in vivo fitness is affected in the N267D variant described here remains to be determined.
The rapid selection of resistance in our patient highlights the risks of tecovirimat monotherapy, especially in the context of prolonged disease and immunosuppression. In such cases, we advocate for surveillance for resistant variants, emphasis on immune reconstitution, monitoring of viral clearance, and strict adherence to infection prevention measures. Additional research is needed on strategies that increase the barrier to resistance, including the use of antiviral combination treatments
(4). Jeremy T. Hua, et al. Engineered StoneAssociated SilicosisA Lethal Variant of an Ancient Disease. JAMA Intern Med. 2023.
Silicosis Among Immigrant Engineered Stone (Quartz) Countertop Fabrication Workers in California
Silicosis is a progressive and irreversible chronic lung disease caused by inhalation of respirable crystalline silica. Although one of the oldest recognized occupational diseases and fully preventable, silicosis is not a disease of the past. Silicosis remains an important cause of chronic respiratory disease worldwide. Silica is one of the most abundant natural minerals, and inhalation of respirable crystalline silica (hereafter silica&) can occur in a wide range of industries, including construction, mining, quarrying, manufacturing, and the production and finishing of stone products, such as kitchen and bathroom countertops.
Engineered stone (also known as artificial or synthetic stone, quartz agglomerate, quartz, or by specific trade names) is an alternative to natural stone and synthetic countertops that is growing in popularity. Over the past decade, outbreaks of severe silicosis have been reported in a number of high-income countries among workers who produce, cut, grind, polish, and install countertops made of engineered stone.1-3 Engineered stone, a hard composite material produced by binding high silicacontent crushed stone with resins and pigments, typically contains over 90% silica, a much higher silica content than natural stone, such as marble (less than 5%) or granite (less than 40%).
Despite the rapid growth in demand for engineered stone countertops, published reports of silicosis cases from the US have been limited.3 Most clinicians, workers, and consumers are unaware of the serious hazards related to engineered stone. In this issue of JAMA Internal Medicine, Fazio and colleagues4 report a California-based case series of 52 engineered stone countertop fabricators with recently diagnosed silicosis. Eight of the cases have been reported previously.3,5 This large case series raises far-reaching concerns about the use of engineered stone products and the effectiveness of regulatory efforts to prevent silicosis.
Similar to previous reports of silicosis in engineered stone workers,1,3,6 the patients in the study by Fazio et al4 were relatively young (median age, 45 years), and many had severe disease. Twenty patients (38%) had advanced silicosis (progressive massive fibrosis with moderate to severely reduced lung function) despite a median of only 15 years of exposure. Ten (19%) had died at the time of the report. Such severe disease in younger workers is rarely seen with traditional& silicosis in other industries, such as construction and quarrying.
The case series also highlights the challenges in diagnosing engineered stoneassociated silicosis, particularly without an occupational history. It is troubling that diagnosis was frequently delayed. Over half the cases were initially diagnosed with other diseases, most commonly with pneumonia or mycobacterial lung infections, diseases that are causally linked to silicosis. Similar to other reports,2,6 autoimmune disease (12%) and autoimmune serologies (antinuclear antibody, 58%; rheumatoid factor, 25%) were common, and they may have contributed to delays in reaching the diagnosis. Chest computed tomography scan findings showed diverse patterns, with a high prevalence of mediastinal/hilar adenopathy (over 80%) and ground glass opacities (37%). The variable chest imaging reflects the broad spectrum of engineered stoneassociated silicosis (including simple silicosis, progressive massive fibrosis, and acute silico-proteinosis), further adding to the challenge of diagnosis. The delays in diagnosis also likely reflect limited clinician awareness of occupational diseases, as well as limited job information in most medical records. Although work is a key social determinant of health, patients are rarely asked about their employment histories.
The findings of Fazio et al4 prompt several key questions. How many undiagnosed cases are there among the approximately 100 000 engineered stone workers in over 8600 fabrication establishments across the US?3 What is the prognosis for these workers? What is the most effective way to address this resurgence of severe silicosis?
Answering these questions is particularly urgent because engineered stone countertops have skyrocketed in popularity in the US (Figure).7 Industry experts anticipate that engineered stone products will overtake all other countertop options by 2024, a remarkable increase for a product that had a less than 5% share of the US market just 15 years ago.7 This shift is due to interrelated factors: a more durable, stain resistant product; an endless choice of shapes, colors, and patterns; declining prices as manufacturing expands in lower-income countries; and limited government oversight.
Current and projected US countertop demands was calculated based on publicly available countertop sales information from the Freedonia Group,7 based on square footage of annual countertop sales. The dashed lines represent projected change in demand.
The best prevalence data for engineered stone silicosis come from Australia. Following alarming outbreaks of severe silicosis similar to the cases reported by Fazio et al,4 the Australian states of Victoria and Queensland implemented mandatory medical surveillance for engineered stone workers.8 At present, nearly 25% of over 1800 engineered stone workers in Australia who have undergone surveillance have been found to have silicosis, and about 15% of those with silicosis have had progressive massive fibrosis.8 Although a smaller fraction (15%) had advanced disease compared with the cases reported by Fazio et al (38%),4 these surveillance findings are disturbing. Since silicosis can progress after the exposure is eliminated, and given the relatively young ages of the workers, both the lifetime prevalence of the disease and its severity are likely to further increase over time.
Surveillance data from the US are more limited. Unlike Australia, there is no systematic medical surveillance of engineered stone workers, despite the more stringent and comprehensive regulations that the Occupational Safety and Health Administration issued in 2016 to prevent silicosis. These updated silica standards for general industry and construction (29 CFR 1910.1053; 29 CFR 1926.1153) lowered the permissible exposure limit for silica and require employers to provide preemployment and periodic medical surveillance of silica-exposed workers.
The report from Fazio et al4 provides insights into the challenges of enforcing government silica standards, including medical surveillance for engineered stone workers. The reported cases were almost entirely among Latino immigrants, the majority of whom were uninsured or underinsured and may have had undocumented immigration status. Across the US, more hazardous jobs are frequently performed by immigrant and other vulnerable workers with limited access to medical care and job protections. Many are hired as independent contractors, an employment status that can circumvent employer responsibilities for medical insurance, workplace protections, surveillance, and workers compensation.
Unlike most states, California has both a strong Department of Public Health and an active Occupational Health Branch with access to multiple information sources, including direct clinician reporting, hospital discharge data, and workplace referral. California is also one of the few states that provides medical insurance for undocumented immigrants. Further, over 70% of the cases in the report by Fazio et al4 were from Los Angeles County, and the majority were identified by a single astute pulmonary clinician at 1 safety net hospital, with only 8 (15%) identified through workplace medical surveillance. This unique constellation of resources and circumstances facilitated the diagnosis and reporting of these 52 cases of silicosis. Most other US states lack comparable resources, and there is no national reporting system for occupational diseases, including silicosis.
Silicosis is an entirely preventable disease. The large number of cases reported by Fazio et al,4 in conjunction with other reports documenting severe disease and high silica exposures in the engineered stone industry,9 demonstrate the inadequacy of current efforts to control silica exposures and protect engineered stone workers. In addition to the high levels of silica dust generated from engineered stone, there may be toxicity related to characteristics of the inhaled dust, such as particle size or composition. Workplace engineering controls and dust suppression methods can reduce airborne silica exposures, along with properly selected and fit-tested respirators. However, there are multiple challenges to implementing such approaches.4 In the case series, many patients worked in shops with fewer than 10 employees, which is common in the industry. Exposure controls are particularly challenging to implement and monitor in small establishments. In addition, the Occupational Safety and Health Administration is severely underresourced, limiting workplace inspections and enforcement. Current efforts to protect workers from engineered stoneassociated silicosis are inadequate.
In addition to primary prevention, there is an urgent need for widespread and systematic medical surveillance of stone fabrication workers, in conjunction with national and state reporting of cases, and appropriate medical care for those identified with disease. Along with increasing awareness among clinicians of this emerging threat, consumers should be informed and aware of the risks that may be inherent in their choice of interior design products. If efforts focused on silica dust control at the workplace and medical surveillance are inadequate in preventing silicosis, elimination of hazardous products and substitution of safer alternatives are the most effective ways to protect workers. In the absence of enhanced primary prevention and medical surveillance, astute clinicians remain the most important resource for vulnerable workers to obtain a correct diagnosis and to receive appropriate medical care.
(5). Sarah J Matthews et al. Atopic eczema in under 12s: diagnosis and managementsummary of updated NICE guidance. BMJ 2023;382:p1538.
What you need to know
Do not offer emollient bath additives to children under 12 years old with atopic eczema
Offer personalised advice to all so that children and carers are educated on how to wash with emollients
Atopic eczema is a common condition in children under 12 years old that can negatively affect quality of life for children and their carers. Emollients form the basis of eczema management and are available as either leave-on preparations (which include lotions, sprays, creams, and ointments), emollient soap substitutes, or emollient bath additives. Management of eczema with emollients includes regularly applying leave-on emollients and washing with leave-on emollients or emollient soap substitutes (box 1).
Tips on emollient safety
Emollients can transfer from skin to clothes, bedding, and bandages, which can then become flammable. Regularly washing clothes and bedding at the highest temperature allowed for the fabric can reduce build-up of emollient, but it does not completely remove it and carers should try to keep children away from fire, flames, and cigarettes
Patients and carers should try to prevent contamination of emollients by either using a pump dispenser or by scooping emollients out of pots using a clean spoon or spatula rather than their hands
Emollients can make the bath or shower slippery. Patients and carers should always use a non-slip bathmat, clean the bath or shower after washing, and dry the bath or shower with paper towels or tissue.
(6). Julio Rosenstock et al. Weekly Icodec versus Daily Glargine U100 in Type 2 Diabetes without Previous Insulin. N Engl J Med. 2023;389:297-308.
Insulin icodec is an investigational once-weekly basal insulin analogue for diabetes management.
We conducted a 78-week randomized, open-label, treat-to-target phase 3a trial (including a 52-week main phase and a 26-week extension phase, plus a 5-week follow-up period) involving adults with type 2 diabetes (glycated hemoglobin level, 7 to 11%) who had not previously received insulin. Participants were randomly assigned in a 1:1 ratio to receive once-weekly insulin icodec or once-daily insulin glargine U100. The primary end point was the change in the glycated hemoglobin level from baseline to week 52; the confirmatory secondary end point was the percentage of time spent in the glycemic range of 70 to 180 mg per deciliter (3.9 to 10.0 mmol per liter) in weeks 48 to 52. Hypoglycemic episodes (from baseline to weeks 52 and 83) were recorded.
Each group included 492 participants. Baseline characteristics were similar in the two groups. The mean reduction in the glycated hemoglobin level at 52 weeks was greater with icodec than with glargine U100 (from 8.50% to 6.93% with icodec [mean change, 1.55 percentage points] and from 8.44% to 7.12% with glargine U100 [mean change, 1.35 percentage points]); the estimated between-group difference (0.19 percentage points; 95% confidence interval [CI], 0.36 to 0.03) confirmed the noninferiority (P0.001) and superiority (P=0.02) of icodec. The percentage of time spent in the glycemic range of 70 to 180 mg per deciliter was significantly higher with icodec than with glargine U100 (71.9% vs. 66.9%; estimated between-group difference, 4.27 percentage points [95% CI, 1.92 to 6.62]; P0.001), which confirmed superiority. Rates of combined clinically significant or severe hypoglycemia were 0.30 events per person-year of exposure with icodec and 0.16 events per person-year of exposure with glargine U100 at week 52 (estimated rate ratio, 1.64; 95% CI, 0.98 to 2.75) and 0.30 and 0.16 events per person-year of exposure, respectively, at week 83 (estimated rate ratio, 1.63; 95% CI, 1.02 to 2.61). No new safety signals were identified, and incidences of adverse events were similar in the two groups.
Glycemic control was significantly better with once-weekly insulin icodec than with once-daily insulin glargine U100. (Funded by Novo Nordisk; ONWARDS 1 ClinicalTrials)
(7). Yves Dauvilliers et al. Oral Orexin Receptor 2 Agonist in Narcolepsy Type 1. N Engl J Med 2023;389:309-321.
Narcolepsy type 1 is caused by severe loss or lack of brain orexin neuropeptides.
We conducted a phase 2, randomized, placebo-controlled trial of TAK-994, an oral orexin receptor 2selective agonist, in patients with narcolepsy type 1. Patients with confirmed narcolepsy type 1 according to clinical criteria were randomly assigned to receive twice-daily oral TAK-994 (30 mg, 90 mg, or 180 mg) or placebo. The primary end point was the mean change from baseline to week 8 in average sleep latency (the time it takes to fall asleep) on the Maintenance of Wakefulness Test (range, 0 to 40 minutes; normal ability to stay awake, ≥20 minutes). Secondary end points included the change in the Epworth Sleepiness Scale (ESS) score (range, 0 to 24, with higher scores indicating greater daytime sleepiness; normal, 10) and the weekly cataplexy rate.
Of the 73 patients, 17 received TAK-994 at a dose of 30 mg twice daily, 20 received 90 mg twice daily, 19 received 180 mg twice daily, and 17 received placebo. The phase 2 trial and an extension trial were terminated early owing to hepatic adverse events. Primary end-point data were available for 41 patients (56%); the main reason for missing data was early trial termination. Least-squares mean changes to week 8 in average sleep latency on the MWT were 23.9 minutes in the 30-mg group, 27.4 minutes in the 90-mg group, 32.6 minutes in the 180-mg group, and 2.5 minutes in the placebo group (difference vs. placebo, 26.4 minutes in the 30-mg group, 29.9 minutes in the 90-mg group, and 35.0 minutes the 180-mg group; P0.001 for all comparisons). Least-squares mean changes to week 8 in the ESS score were 12.2 in the 30-mg group, 13.5 in the 90-mg group, 15.1 in the 180-mg group, and 2.1 in the placebo group (difference vs. placebo, 10.1 in the 30-mg group, 11.4 in the 90-mg group, and 13.0 in the 180-mg group). Weekly incidences of cataplexy at week 8 were 0.27 in the 30-mg group, 1.14 in the 90-mg group, 0.88 in the 180-mg group, and 5.83 in the placebo group (rate ratio vs. placebo, 0.05 in the 30-mg group, 0.20 in the 90-mg group, and 0.15 in the 180-mg group). A total of 44 of 56 patients (79%) receiving TAK-994 had adverse events, most commonly urinary urgency or frequency. Clinically important elevations in liver-enzyme levels occurred in 5 patients, and drug-induced liver injury meeting Hys law criteria occurred in 3 patients.
In a phase 2 trial involving patients with narcolepsy type 1, an orexin receptor 2 agonist resulted in greater improvements on measures of sleepiness and cataplexy than placebo over a period of 8 weeks but was associated with hepatotoxic effects. (Funded by Takeda Development Center Americas)
(8). Deborah Schrag et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N Engl J Med. 2023;389:322-334
Pelvic radiation plus sensitizing chemotherapy with a fluoropyrimidine (chemoradiotherapy) before surgery is standard care for locally advanced rectal cancer in North America. Whether neoadjuvant chemotherapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) can be used in lieu of chemoradiotherapy is uncertain.
We conducted a multicenter, unblinded, noninferiority, randomized trial of neoadjuvant FOLFOX (with chemoradiotherapy given only if the primary tumor decreased in size by 20% or if FOLFOX was discontinued because of side effects) as compared with chemoradiotherapy. Adults with rectal cancer that had been clinically staged as T2 node-positive, T3 node-negative, or T3 node-positive who were candidates for sphincter-sparing surgery were eligible to participate. The primary end point was disease-free survival. Noninferiority would be claimed if the upper limit of the two-sided 90.2% confidence interval of the hazard ratio for disease recurrence or death did not exceed 1.29. Secondary end points included overall survival, local recurrence (in a time-to-event analysis), complete pathological resection, complete response, and toxic effects.
From June 2012 through December 2018, a total of 1194 patients underwent randomization and 1128 started treatment; among those who started treatment, 585 were in the FOLFOX group and 543 in the chemoradiotherapy group. At a median follow-up of 58 months, FOLFOX was noninferior to chemoradiotherapy for disease-free survival (hazard ratio for disease recurrence or death, 0.92; 90.2% confidence interval [CI], 0.74 to 1.14; P=0.005 for noninferiority). Five-year disease-free survival was 80.8% (95% CI, 77.9 to 83.7) in the FOLFOX group and 78.6% (95% CI, 75.4 to 81.8) in the chemoradiotherapy group. The groups were similar with respect to overall survival (hazard ratio for death, 1.04; 95% CI, 0.74 to 1.44) and local recurrence (hazard ratio, 1.18; 95% CI, 0.44 to 3.16). In the FOLFOX group, 53 patients (9.1%) received preoperative chemoradiotherapy and 8 (1.4%) received postoperative chemoradiotherapy.
In patients with locally advanced rectal cancer who were eligible for sphincter-sparing surgery, preoperative FOLFOX was noninferior to preoperative chemoradiotherapy with respect to disease-free survival. (Funded by the National Cancer Institute; PROSPECT ClinicalTrials)
(9). Jesíºs San-Miguel et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J Med. 2023;389:335-347.
Ciltacabtagene autoleucel (cilta-cel), a B-cell maturation antigen (BCMA)directed CAR T-cell therapy, is effective in heavily pretreated patients with relapsed or refractory multiple myeloma. We investigated cilta-cel in earlier treatment lines in patients with lenalidomide-refractory disease.
In this phase 3, randomized, open-label trial, we assigned patients with lenalidomide-refractory multiple myeloma to receive cilta-cel or the physicians choice of effective standard care. All the patients had received one to three previous lines of treatment. The primary outcome was progression-free survival.
A total of 419 patients underwent randomization (208 to receive cilta-cel and 211 to receive standard care). At a median follow-up of 15.9 months (range, 0.1 to 27.3), the median progression-free survival was not reached in the cilta-cel group and was 11.8 months in the standard-care group (hazard ratio, 0.26; 95% confidence interval [CI], 0.18 to 0.38; P0.001). Progression-free survival at 12 months was 75.9% (95% CI, 69.4 to 81.1) in the cilta-cel group and 48.6% (95% CI, 41.5 to 55.3) in the standard-care group. More patients in the cilta-cel group than in the standard-care group had an overall response (84.6% vs. 67.3%), a complete response or better (73.1% vs. 21.8%), and an absence of minimal residual disease (60.6% vs. 15.6%). Death from any cause was reported in 39 patients and 46 patients, respectively (hazard ratio, 0.78; 95% CI, 0.5 to 1.2). Most patients reported grade 3 or 4 adverse events during treatment. Among the 176 patients who received cilta-cel in the as-treated population, 134 (76.1%) had cytokine release syndrome (grade 3 or 4, 1.1%; no grade 5), 8 (4.5%) had immune effector cellassociated neurotoxicity syndrome (all grade 1 or 2), 1 had movement and neurocognitive symptoms (grade 1), 16 (9.1%) had cranial nerve palsy (grade 2, 8.0%; grade 3, 1.1%), and 5 (2.8%) had CAR-Trelated peripheral neuropathy (grade 1 or 2, 2.3%; grade 3, 0.6%).
A single cilta-cel infusion resulted in a lower risk of disease progression or death than standard care in lenalidomide-refractory patients with multiple myeloma who had received one to three previous therapies. (Funded by Janssen and Legend Biotech; CARTITUDE-4 ClinicalTrials.)
(10). Steven K. Grinspoon et al. Pitavastatin to Prevent Cardiovascular Disease in HIV Infection. 2023
The risk of cardiovascular disease is increased among persons with human immunodeficiency virus (HIV) infection, so data regarding primary prevention strategies in this population are needed.
In this phase 3 trial, we randomly assigned 7769 participants with HIV infection with a low-to-moderate risk of cardiovascular disease who were receiving antiretroviral therapy to receive daily pitavastatin calcium (at a dose of 4 mg) or placebo. The primary outcome was the occurrence of a major adverse cardiovascular event, which was defined as a composite of cardiovascular death, myocardial infarction, hospitalization for unstable angina, stroke, transient ischemic attack, peripheral arterial ischemia, revascularization, or death from an undetermined cause.
The median age of the participants was 50 years (interquartile range, 45 to 55); the median CD4 count was 621 cells per cubic millimeter (interquartile range, 448 to 827), and the HIV RNA value was below quantification in 5250 of 5997 participants (87.5%) with available data. The trial was stopped early for efficacy after a median follow-up of 5.1 years (interquartile range, 4.3 to 5.9). The incidence of a major adverse cardiovascular event was 4.81 per 1000 person-years in the pitavastatin group and 7.32 per 1000 person-years in the placebo group (hazard ratio, 0.65; 95% confidence interval [CI], 0.48 to 0.90; P=0.002). Muscle-related symptoms occurred in 91 participants (2.3%) in the pitavastatin group and in 53 (1.4%) in the placebo group; diabetes mellitus occurred in 206 participants (5.3%) and in 155 (4.0%), respectively.
Participants with HIV infection who received pitavastatin had a lower risk of a major adverse cardiovascular event than those who received placebo over a median follow-up of 5.1 years. (Funded by the National Institutes of Health and others; REPRIEVE ClinicalTrials)
(11). Thibault Kerdiles et al. Superior Mesenteric Artery Syndrome. N Engl J Med 2023;389:359.
A 26-year-old man from Somalia presented to the emergency department with a 5-month history of dry cough, night sweats, and unintentional weight loss of 18 kg. During this period, epigastric pain and episodes of postprandial vomiting had also developed. His body-mass index (the weight in kilograms divided by the square of the height in meters) was 11.
Physical examination was notable for cachexia and abdominal distention with diffuse, mild tenderness to palpation.
On the basis of chest imaging and sputum studies, a diagnosis of pulmonary tuberculosis was made. Intravenous antituberculous treatment was initiated, but the patient continued to have postprandial vomiting. Subsequent contrast-enhanced computed tomography of the abdomen showed a paucity of mesenteric and subcutaneous fat, dilatation of the stomach (Panel A, asterisk), and compression of the duodenum between the superior mesenteric artery and the aorta, with an aortomesenteric distance of 3 mm (reference range, 10 to 20) (Panel A, arrow) and an aortomesenteric angle of 7 degrees (reference range, 45 to 60) (Panel B).
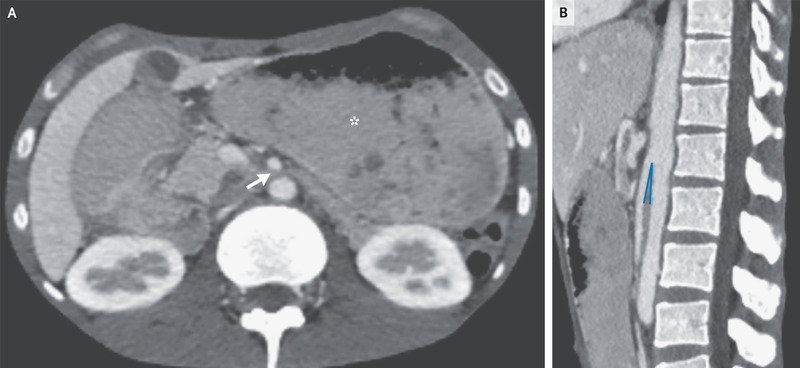
A diagnosis of superior mesenteric artery syndrome due to cachexia from tuberculosis was made. A nasogastric tube was inserted and parenteral nutrition was initiated; however, the patient opted to leave the hospital after 2 weeks and was lost to follow-up.
(12). Image Challenge. NEJM, 2023
A 13-year-old boy from Mali was referred to the pediatric urology clinic with a 3-month history of gross hematuria. He reported no fevers, flank pain, or dysuria. A physical examination was normal. Laboratory studies showed normal kidney function and an absolute eosinophil count of 2660 per cubic millimeter (reference range, 40-200). A urinalysis showed hematuria and pyuria, and a urine culture was negative. Microsopic examination of the urine is shown. What is the most likely diagnosis?
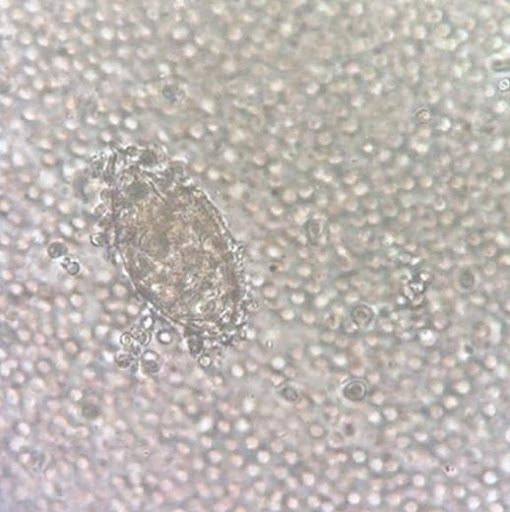
- Balantidium coli
- Schistosoma hematobium
- Schistosoma mansoni
- Strongyloides stercoralis
- Trichomonas vaginalis
The correct answer is Schistosoma hematobium, a parasite prevalent in Africa and the Middle East. The microscopic examination of urine showed oval-shaped parasite eggs with a terminal spine. Histopathological examination of a bladder-biopsy specimen showed acute and chronic papillary and polypoid cystitis, and no dysplasia. Treatment with praziquantel was administered, and symptoms resolved one month after initiation.
(13). Daniel Sokol. In praise of civility among clinicians. BMJ 2023;382:p1700
The very first textbook of medical ethicsThomas Percivals Medical Ethics, published in 1803emphasised the importance of honourable conduct and mutual respect. Percival acknowledged the possibility of controversy among doctors but warned that this should be resolved within the profession. It shouldnt spill into the public domain, he said, for fear of damaging the reputation of the doctors concerned and the general credit of the faculty.&
In an 1892 address the Canadian physician William Osler spoke of the wrangling and unseemly disputes which have too often disgraced our profession.& He believed that, in most cases, these disputes arose on the one hand, from the morbid sensitiveness to the confession of error, and, on the other, from a lack of brotherly consideration, and a convenient forgetfulness of our own failing.&
In 1902 James Sprague, in his Medical Ethics and Cognate Subjects, also lamented doctors who spoke ill of each other: There is nothing more unpleasant to my ears than to hear my medical brethren talk disparagingly of each other.&
Half a century later, Stephen Hadfields Law and Ethics for Doctors (1958) included a chapter on the ethical obligations of doctors to one another. The chapter started thus: In the interests of themselves and of their patients, doctors should support their colleagues at all times and preserve proper ethical relationships with them.&
Prone to amplification
This advice lives on today through the World Medical Associations Declaration of Geneva, which states, I will give to my colleagues the respect and gratitude that is their due,& and the UKs General Medical Council, which instructs doctors to treat your colleagues fairly and with respect.&
Applied to doctors who use social media, the first rule is that everything you post online is a public statement prone to amplification, so be highly sensitive to how you might come across. The second rule is to treat clinical colleagues with respect and dignity, even if you disagree with them. If this proves too difficult, its often best to say nothing at all in public.
Footnotes
Daniel Sokol is a medical ethicist and barrister. He is the author of Tough Choices: Stories from the Front Line of Medical Ethics (2018).
References
- Percival T. Medical ethics. Classics of Medicine Library, 1985.
- Osler W. Teacher and student. In: Aequanimitas with other addresses to medical students, nurses and practitioners of medicine.2nd ed. P Blakistons Son & Co, 1910: 21-43.Google Scholar
- Sprague J. Medical ethics and cognate subjects.Charles Sparling, 1902.Google Scholar
- Hadfield S. Law and ethics for doctors.Eyre & Spottiswoode, 1958.Google Scholar
- World Medical Association. Declaration of Geneva. 2017. https://www.wma.net/policies-post/wma-declaration-of-geneva/
- General Medical Council. Leadership and management for all doctors: working with colleagues. 2012. https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/leadership-and-management-for-all-doctors/working-with-colleagues#respect-for-colleagues
(14). Stuart Stewart et al. Managing raised ferritin in primary care. BMJ 2023;382:e076750
What you need to know
- Raised levels of ferritin can be associated with several serious underlying conditions and should be investigated appropriately
- Determine whether raised ferritin reflects iron overload or another disease process
- Initial tests for investigating raised ferritin in primary care are fasting transferrin saturation, full blood count, liver blood tests, and C reactive protein
- Assess patients for organ damage associated with iron overload to determine further investigations, management, and whether the patient needs to be referred to secondary care
- Haemochromatosis is a common genetic condition that can cause iron overload, and primary care clinicians can order HFE gene mutation analysis to diagnose the condition
(15). Ali A Asadi-Pooya. Adult epilepsy. Lancet 2023;402(10399):P412-424.
Epilepsy is a common medical condition that affects people of all ages, races, social classes, and geographical regions. Diagnosis of epilepsy remains clinical, and ancillary investigations (electroencephalography, imaging, etc) are of aid to determine the type, cause, and prognosis. Antiseizure medications represent the mainstay of epilepsy treatment: they aim to suppress seizures without adverse events, but they do not affect the underlying predisposition to generate seizures. Currently available antiseizure medications are effective in around two-thirds of patients with epilepsy. Neurosurgical resection is an effective strategy to reach seizure control in selected individuals with drug-resistant focal epilepsy. Non-pharmacological treatments such as palliative surgery (eg, corpus callosotomy), neuromodulation techniques (eg, vagus nerve stimulation), and dietary interventions represent therapeutic options for patients with drug-resistant epilepsy who are not suitable for resective brain surgery.
(16). Hetty E Carraway. Are we ready to ring in a new upfront therapy in lower-risk myelodysplastic syndromes? Lancet 2023;402(10399):P348-350.
Fatigue due to anaemia is one of the most common symptoms that brings patients diagnosed with lower-risk myelodysplastic syndromes to medical attention. As a result, the goals in treating patients with lower-risk myelodysplastic syndromes are often focused on improving cytopenias and quality of life. Erythropoiesis-stimulating agents (ESAs), such as recombinant erythropoietin (ie, epoetin alfa and darbepoetin alfa), are used for transfusion-dependent anaemia, particularly in patients with low serum erythropoietin (500 U/L) and a red blood cell transfusion requirement of less than 2 red blood cell units per month. Response to ESA-based therapy can be as high as 70%, 1 but if serum erythropoietin is higher than 500 U/L, the expected response rate (defined as packed red blood cell transfusion independence or a rise in haemoglobin ≥15 g/L, or both for at least an 816-week time period) 2 , 3 is lower than 10%. 1 In several phase 3 randomised trials comparing erythropoietin versus supportive care, the expected response rate was as high as 47% with erythropoietin. 4 , 5 Similar results were seen in a phase 3 placebo-controlled study of darbepoetin alfa with a response rate of 35%. 6 A pooled analysis of more than 500 patients with lower-risk myelodysplastic syndromes revealed that the majority of clinical responses to ESA therapies occurred within 3 months of treatment and had a median duration of 17 months. 7 In April, 2020, luspaterceptan erythroid maturation agent with a mechanism of action distinct from ESA therapywas approved by the US Food and Drug Administration for patients with lower-risk myelodysplastic syndromes (with the presence of ring sideroblasts and or SF3B1 mutation) who were transfusion dependent with disease refractory to or unlikely to respond to ESA-based therapy. Luspatercept binds to select transforming growth factor- superfamily ligands and decreases Smad-2/3 signalling. It is this inhibitory effect on Smad-2/3 signalling that enables late stage erythroblast differentiation. 8 The PACE study established luspatercept to have efficacy in the treatment of red blood cell transfusion dependent anaemia in patients with lower-risk myelodysplastic syndromes with disease that was relapsed or refractory to ESA-based therapy; with low serum erythropoietin or an SPF3B1 mutation (and or the presence of ring sideroblasts) being predictive of success. 9 This led to the MEDALIST trial, a phase 3 randomised study evaluating luspatercept versus placebo in transfusion-dependent, lower-risk myelodysplastic syndromes (either relapsed, refractory, or unlikely to respond to ESAs). Presence of either 15% or higher ring sideroblasts or 5% or higher ring sideroblasts plus an SF3B1 mutation was required for eligibility. An erythroid response during the first 24 weeks occurred in 81 (53%) of 153 patients in the luspatercept group versus nine (12%) of 76 in the placebo group, and transfusion independence lasting for greater than 8 weeks was achieved in 58 (38%) versus ten (13%), respectively (p0001). The median duration of response in the luspatercept-treated group was 306 weeks
(17). Meletios A Dimopoulos et al. Management of multiple myeloma-related renal impairment: recommendations from the International Myeloma Working Group. Lancet 2023;24(7):E293-E311.
Here, the International Myeloma Working Group (IMWG) updates its clinical practice recommendations for the management of multiple myeloma-related renal impairment on the basis of data published until Dec 31, 2022. All patients with multiple myeloma and renal impairment should have serum creatinine, estimated glomerular filtration rate, and free light chains (FLCs) measurements together with 24-h urine total protein, electrophoresis, and immunofixation. If non-selective proteinuria (mainly albuminuria) or involved serum FLCs value less than 500 mg/L is detected, then a renal biopsy is needed. The IMWG criteria for the definition of renal response should be used. Supportive care and high-dose dexamethasone are required for all patients with myeloma-induced renal impairment. Mechanical approaches do not increase overall survival. Bortezomib-based regimens are the cornerstone of the management of patients with multiple myeloma and renal impairment at diagnosis. New quadruplet and triplet combinations, including proteasome inhibitors, immunomodulatory drugs, and anti-CD38 monoclonal antibodies, improve renal and survival outcomes in both newly diagnosed patients and those with relapsed or refractory disease. Conjugated antibodies, chimeric antigen receptor T-cells, and T-cell engagers are well tolerated and effective in patients with moderate renal impairment.
18.
Bacterial infection linked to endometriosis
Priya Venkatesan, Open AccessPublished:July 18, 2023DOI:https://doi.org/10.1016/S2666-5247(23)00221-5, https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(23)00221-5/fulltext
A translational study has suggested that Fusobacterium infection of the endometrium might contribute to the pathogenesis of endometriosis.
Endometriosis is a chronic, painful disease that affects around 10% of women of reproductive age worldwide. Characterised by endometrial-like tissue growing outside the uterus, the disease leads to systemic inflammation, scar tissue, and lesions forming in the pelvis and other areas of the body, causing multiple symptoms including chronic pelvic pain, heavy bleeding during or in between menstruations, infertility, fatigue, and depression. Treatment options are few and only manage the symptoms.
Part of the problem of finding treatment options is that the cause of endometriosis is not fully known. Although retrograde menstruation is generally accepted as a cause of endometriosis, most women experience retrograde menstruation yet only 10% develop endometriosis, suggesting that other factors might contribute to its development. Dominique de Ziegler (Department of Obstetrics and Gynecology and Reproductive Medicine, Hopital Foch -Universit de Paris Ouest UVSQ, Paris, France) commented There has been mounting evidence that the primary mechanism in some or all cases of endometriosis resides in alterations of the endometrium stemming from inflammation.& Stromal fibroblast proliferation and migration are key contributors to the progression of endometriosis, and inflammation has been shown to trigger the transition of quiescent fibroblasts to activated myofibroblasts in other chronic conditions such as fibrosis. Additionally, as the incidence of endometritis (ie, a bacterial infection of the endometrium causing inflammation) is markedly increased in women with endometriosis, an infectious aetiology of endometriosis is conceivable. Five bacterial genera are thought to be significantly increased in the endometria of patients with endometriosis compared with individuals without endometriosis, including Erysipelothrix and Fusobacterium.
Ayako Muraoka (Division of Cancer Biology, Nagoya University Graduate School of Medicine, Nagoya, Japan) and colleagues did a study using uterine tissue samples from 79 patients in two Japanese hospitals to investigate whether the types of endometrial fibroblasts differed between people with and without endometriosis, and whether fibroblasts from individuals with endometriosis were associated with the presence of bacteria.
qPCR analysis showed that Erysipelothrix was not abundant in the endometrial tissue from study participants. However, Fusobacterium infiltration, mostly Fusobacterium nucleatum, was significantly more frequent in endometrial and endometriotic tissues from patients with endometriosis (27 [643%] of 42) than from the controls (three [71%] of 42; p001). Muraoka and colleagues found that the protein transgelin (TAGLN), linked to increased cell motility and migration, was up-regulated in fibroblasts from patients with endometriosis and that TAGLN was specifically expressed by these fibroblasts. Immunohistochemical analyses showed that Fusobacterium infection of endometrial cells activated transforming growth factor signalling (known to play a major role in endometriosis), leading to quiescent fibroblasts transitioning to TAGLN-positive myofibroblasts with the ability to proliferate, adhere, and migrate in vitro.
In subsequent in-vivo experiments in a mouse model, Muraoka and colleagues showed that inoculation of tissue from F nucleatum-infected uteri from donor mice caused the formation of multiple endometriotic lesions in recipient mice, whereas tissue from uninfected uteri did not cause endometriosis. Infection with Lactobacillus iners, a major bacterium in the vaginal microbiota of reproductive-age women, did not cause the development of endometriotic lesions. And mice receiving endometrial tissue from donor mice infected with F nucleatum that had been treated with metronidazole and chloramphenicol developed fewer endometriotic lesions than mice receiving tissue from infected mice that were not treated with antibiotics (p001).
Muraoka and colleagues study is the first to suggest a link between infection and endometriosis; although a previous study had shown that treatment with metronidazole reduced the growth and progression of endometriotic lesions in a mouse model, any relevance to human endometriosis was unclear. Co-author Yutaka Kondo (Division of Cancer Biology, Nagoya University Graduate School of Medicine) commented Our findings suggest that Fusobacterium infection may contribute to the pathogenesis of endometriosis and that antibiotic treatment to eradicate endometrial infection should be further studied. Therefore, the next important requirement is clinical trials of antibiotic treatment for endometriosis patients in a big cohort.& de Ziegler said The primary mechanism causing the inflammatory alterations identified in the eutopic endometrium and prone to causing endometriosis are unknown. The study by Muraoka et al is of prime importance; the identification of Fusobacterium infection is clearly a plausible path that needs to be further investigated.& She added At this stage, studies are urgently needed for treating endometriosis.&
(19). Sigismond Lasocki et al. Ferric derisomaltose and tranexamic acid, combined or alone, for reducing blood transfusion in patients with hip fracture (the HiFIT trial): a multicentre, 22 factorial, randomised, double-blind, controlled trial. 2023.
Anaemia and blood transfusion are associated with poor outcomes after hip fracture. We evaluated the efficacy of intravenous iron and tranexamic acid in reducing blood transfusions after hip fracture surgery.
In this double-blind, randomised, 22 factorial trial, we recruited adults hospitalised for hip fractures in 12 medical centres in France who had preoperative haemoglobin concentrations between 95 and 130 g/dL. We randomly allocated participants (1:1:1:1), via a secure web-based service, to ferric derisomaltose (20 mg/kg intravenously) and tranexamic acid (1 g bolus followed by 1 g over 8 h intravenously at inclusion and 3 g topically during surgery), iron plus placebo (normal saline), tranexamic acid plus placebo, or double placebo. Unmasked nurses administered study drugs; participants and other clinical and research staff remained masked to treatment allocation. The primary outcome was the percentage of patients transfused during hospitalisation (or by day 30). The primary analysis included all randomised patients. This study is registered on ClinicalTrials.gov (NCT02972294) and is closed to new participants.
Of 413 patients (51104 years old, median [IQR] 86 [7891], 312 [76%] women, 101 [24%] men), 104 received iron plus tranexamic acid, 103 iron plus placebo, 103 tranexamic acid plus placebo, and 103 double placebo between March 31, 2017 and June 18, 2021 (study stopped early for efficacy after the planned interim analysis done on the first 390 patients included on May 25, 2021). Data for the primary outcome were available for all participants. Among patients on double placebo, 31 (30%) were transfused versus 16 (15%) on both drugs (relative risk 051 [983% CI 027097]; p=0012). 27 (26%) participants on iron (081 [050129]; p=028) and 28 (27%) on tranexamic acid (085 [054133]; p=039) were transfused. 487 adverse events were reported with similar event rates among the groups; among prespecified safety endpoints, severe postoperative anaemia (haemoglobin 8 g/dL) was more frequent in the double placebo group. Main common adverse event were sepsis, pneumonia, and urinary infection, with similar rates among all groups.
In patients hospitalised for hip fracture surgery with a haemoglobin concentration 95130 g/dL, preoperative infusion of ferric derisomaltose plus tranexamic acid reduced the risk of blood transfusion by 50%. Our results suggest that combining treatments from two different pillars improves patient blood-management programmes. Either treatment alone did not reduce transfusion rates, but we might not have had the power to detect it.
(20). Catherine A Cluver et al. Early delivery for pre-eclampsia might save lives in low-income and middle-income settings. Lancet 2023;402(10399):P350-352.
Pre-eclampsia is a leading contributor to maternal and perinatal mortality worldwide, with the burden being disproportionately borne by those in low-income and middle-income countries (LMICs). Despite this disparity, most research in pre-eclampsia is done in high-income settings. An example is identifying the optimal timing of delivery for late preterm pre-eclampsia. Here, clinicians find themselves balancing risks. There is the potential hazard to the mother of continuing the pregnancy with a progressive disease for which the only cure is delivery, and there are competing risks for the fetus: the ongoing exposure to a potentially hostile in-utero environment versus the short-term and long-term consequences of premature birth. The dilemma regarding timing of birth is particularly pertinent for pre-eclampsia because maternal and placental vascular dysfunction can compromise both maternal and fetal wellbeing.
And what of life beyond infancy? Doubts about the effect of late preterm birth on long-term neurodevelopmental outcomes in late preterm pre-eclampsia have been recently allayed,1, 2, 3 which leaves the focus on short-term outcomes.
In high-income settings, answers regarding planned delivery versus expectant management have emerged from well conducted randomised controlled trials. A 2022 individual participant data meta-analysis4 highlights the fine balance of maternal versus perinatal benefits. Planned delivery from 34 weeks gestation in women with pre-eclampsia significantly reduces the risk of maternal adverse events and the chance of the infant being born small for their gestational age. Balanced against this result is the increase in the composite perinatal outcome, mostly driven by short-term neonatal respiratory morbidity.4
Importantly, this individual participant data meta-analysis4 and the preceding Cochrane review5 included only studies done in high-income settings. Whether these findings can be extrapolated to LMICs is crucial, given the low availability of facilities providing advanced-level care for both the mother and infant. The greatest maternal benefit of planned delivery will probably be found in these settings. Yet the added burden of premature births might be unsustainable in resource-constrained neonatal settings.
In this issue of The Lancet, Alice Beardmore-Gray and colleagues6 evaluate whether planned delivery between 34+0 and 36+6 weeks gestation could reduce maternal mortality and morbidity without increasing perinatal complications in LMICs. This impressive, randomised controlled trial (CRADLE 4) enrolled 565 women from nine sites across India and Zambia, including women from both rural and urban settings. Among the 565 enrolled women, 284 were allocated to receive planned delivery and 281 to receive expectant management. The mean maternal age was 28 years, and 28% of these women did not have more than a primary education level. 72% of participants were Black, and 28% were south Asian. Sobering statistics reported in this study highlight the burden of complications in LMICs. 33 (6%) women had a serious adverse event, comprising four maternal deaths, 14 neonatal deaths, and 15 stillbirths, very different outcomes to the studies in high-income settings.4
The CRADLE 4 trial found that planned delivery resulted in a non-significant decreased incidence of the primary maternal composite outcome, which included maternal mortality or multi-organ morbidity (154 [55%] for planned delivery vs 168 [60%] for expectant management; adjusted risk ratio [aRR] 091, 95% CI 079 to 105). Although this composite result failed to reach statistical significance, severe maternal hypertension was significantly reduced (123 [44%] for planned delivery vs 146 [52%] for expectant management, aRR 083, 95% CI 070 to 099). This reduction is an important outcome since severe hypertension itself is associated with adverse maternal and perinatal outcomes,7 including stroke risk.8, 9 Planned delivery did not increase the primary perinatal outcome, which included stillbirth, neonatal death, or neonatal unit admission for more than 48 h (58 [19%] for planned delivery vs 67 [22%] for expectant management, adjusted risk difference for non-inferiority 339%, 90% CI 867 to 190; p00001). Indeed, there was a significant decrease in stillbirths in women who had a planned delivery (3 [10%] for planned delivery vs 12 [40%] for expectant management, aRR 025, 95% CI 007 to 087), although the difference in time from randomisation to delivery was only 288 days. Birthweight centile was also higher among infants born to the planned delivery group than those to the expectant management group, highlighting the potential fetal risks of increasing gestation when severe placental disease is present. Reassuringly, the number of infants needing to be admitted for neonatal care or respiratory support did not differ between the two groups, and neonatal length of stay was comparable.
Strengths include that the multi-site study was done in two continents, increasing generalisability. There was an impressively low loss to follow-up rate of 02%. A feasibility and acceptability study included perspectives from women, their partners, and caregivers, informing both trial design and implementation.10, 11 The authors plan a formal health-care resource analysis and have collected qualitative data exploring womens experiences of the trial. A limitation is the short follow-up of the women and their infants, as they were only followed up until their discharge home. A longer follow-up, to at least 6 weeks after the expected due date, could have been important to determine if there were late complications at home in either group.
This study provides important information for parents and clinicians in LMICs making decisions about the timing of birth in late preterm pre-eclampsia. Planned delivery is safe to offer; maternal benefit is not achieved at the expense of increasing perinatal risk or increasing demands on finite neonatal resources. Importantly, planned delivery is accompanied by an encouraging reduction in stillbirth, with the number of planned deliveries needed to treat to prevent one stillbirth only 33. Planned delivery in late preterm pre-eclampsia is therefore an important intervention to reduce global maternal and perinatal morbidity.
(21). Maxime J Billick et al. HIV prevention with postexposure prophylaxis-in-pocket (PIP). BMJ 2023;382:e076016
What you need to know
Postexposure prophylaxis-in-pocket (PIP) is an HIV prevention strategy that may be particularly suitable for people who have only a low number of high risk, often unanticipated, HIV exposures per year (such as 0-4 per year)
Prescribing postexposure PIP involves proactively providing 28 days of guideline-approved HIV postexposure prophylaxis so that people may self initiate medications after a potential HIV exposure
Evaluate HIV risk at follow-up visits as people may change their preferred HIV prevention modality (for example, between postexposure and pre-exposure prophylaxis) as their needs and circumstances evolve.
(22). Christopher M Booth et al. Common Sense Oncology: outcomes that matter. 2023.
Oncology needs a recalibrated approach that is more patient centred and prioritises equitable cancer care. An approach that prioritises patients needs with treatments that improve survival and quality of life, promotes informed decision making, and ensures these treatments are accessible to all patients.
For both patients and clinicians, cancer treatment decisions are increasingly complicated. While some cancer treatments provide large benefits, many new approved treatments do not help patients live longer or better.1, 2 All cancer treatments have side-effects, can cause substantial financial burden, and can result in lost time for patients spent in hospital rather than with friends and family. Thus, it is important to not only study and promote treatments that improve survival or quality of life (or both), but also to identify treatments that do not. Cancer systems now face a troubling paradox. In some circumstances there is substantial overuse of treatments with very small benefits, and at the same time many patients worldwide do not have access to the treatments that can make a very meaningful difference in their lives.
How have these problems arisen in modern cancer care? The reasons are multifactorial, but one key factor is the shift over the past few decades from predominantly publicly funded clinical trials designed to answer questions important to patients, to industry funded trials designed to achieve regulatory approval or commercial advantage.1 Often the goal of improving and lengthening the lives of patients and that of making a profit for commercial organisations are not concordant. Industrys control of the research agenda has created a system that is predominantly focused on new cancer medicines at the expense of investigating new approaches to surgery, radiotherapy, palliative care, and prevention. This model is problematic for several reasons. Surgery and radiotherapy cure many more patients than cancer medicines, yet receive much less funding for research and delivery of care. Moreover, in many parts of the world the majority of individuals diagnosed have incurable cancer, yet lack access to adequate pain relief and palliative care.
Commercial interests, rather than patient interests, often drive cancer care and research, as seen by the mismatch between research spending on some cancers and their associated mortality and societal impact.3 In some countries, new cancer medicines cost more than US$200 000 per year, including those that do not help patients live longer.4, 5 A substantial proportion of industry revenue is used for marketing campaigns to influence patients, policy makers, and oncologists, irrespective of clinical need.6 Industry marketing campaigns and media reports often hype marginal treatments, which contribute to overuse of cancer treatments with small or negligible benefits.7
Another factor that contributes to these problems is the absence of clear communication regarding the magnitude of benefit and risks associated with therapies. In the context of incurable cancer it is difficult for both oncologists and patients to balance hope with reality when discussing prognosis and treatment options. Patients and clinicians often feel compelled to do something when faced with progressive disease, even if that something has minimal benefit and causes side-effects. Clear and compassionate communication is necessary to ensure that patients make informed treatment choices, supported with honestly reported, evidence-based guidance from health-care teams that match individual goals and values.
Patients deserve better information and better care. To achieve this, paradigm shifts will be needed in education, research design and investment, policy, media and communication, and delivery of care.8 In April, 2023, global oncologists, academics, and patient advocates met at Queens University (Kingston, ON, Canada). The objectives of this meeting were to establish core tenets to guide development of a patient-centred Common Sense Oncology (CSO) movement, develop goals and an action plan, and disseminate CSO guiding principles so that oncology trials and treatments are focused on outcomes that matter.
The mission, vision, and guiding principles of CSO are shown in the panel. CSO will focus initially on three pillars: evidence generation, evidence interpretation, and evidence communication. This work will be patient centred and emphasise health equity. CSO projects will seek solutions for problems with measurable targets to influence cancer research, education, delivery of care, and policy.
Panel
Common Sense Oncology: outcomes that matter
Mission
To ensure that cancer care focuses on outcomes that matter to patients
Vision
Patients have access to cancer treatments that provide meaningful improvements in outcomes that matter, irrespective of where they live or their health system. To realise this vision, we aspire that:
Patient outcomes that matter must be at the centre of every drug registration trial; and patient outcomes that matter should be the standard for every drug regulatory decision
Reporting of trials is transparent and uses language that can be understood clearly by oncologists and patients
Patients receive clear communication regarding treatment options that enables them to make informed decisions that are aligned with their personal goals and values
The only treatments that are registered, reimbursed, and recommended are ones that meaningfully improve patients lives
Common Sense Oncology that is grounded in evidence-based medicine and critical appraisal becomes a core curricular component for oncology training programmes
Health systems invest in both developing new treatments and ensuring that patients have access to and benefit from proven effective treatments
Guiding principles
1. Access to quality cancer care is a basic human rightno patient should be denied access to effective therapy or forced into financial catastrophe to access meaningful cancer care
2. Patient and societal needs should drive cancer research and delivery of care
3. Patient and public involvement is essential when making policy decisions
4. Patients should expect that recommended cancer treatments meaningfully improve their survival or quality of life
5. Shared decision making between patients and oncologists should be based on patient values and grounded in evidence-based medicine and critical appraisal
6. Cancer treatments should be fairly priced for the context in which they are used
7. Equity in access to high quality care should be prioritised as much as innovation and new treatments
8. Comprehensive patient-centred cancer care includes timely integration of psychosocial oncology, survivorship, and palliative care
The first pillar is evidence generation, which aims to ensure that clinical trials use and report outcomes that matter to patients. The randomised controlled trial (RCT) remains the gold standard to evaluate efficacy of new cancer therapies. Although meaningful improvements in patient outcome have come from pivotal trials, there are growing concerns about problems in design and reporting of some RCTs.9 This work stream will seek to offer solutions to improve trial design and reporting to ensure they prioritise outcomes that matter to patients.
The second pillar is evidence interpretation, which aims to foster critical thinking by clinicians. To assist patients in decision making, oncologists must be well-trained in critical appraisal. Individual oncologists and guideline committees should not recommend treatments that are based on poorly designed or poorly reported trials that show marginal benefits. This work stream will aim to empower oncologists to make sound clinical decisions aligned with outcomes that matter to patients.
The third pillar is evidence communication, which aims to improve patient, public, and policy maker understanding of cancer treatment options. The clinical and research communities in oncology must communicate clearly with all stakeholders in the cancer system. Lack of clear communication can lead to unrealistic expectations among patients and hype within oncology societies and the media; this in turn can drive promotion of treatments that provide marginal clinical benefits to patients.10 This work stream will look at ways of facilitating better informed decision making with patients; engagement with policy makers; and work with journalists to ensure that media reports are balanced, contextualised, and less sensational.
CSO will promote interventions that measurably improve the lives of patients. We will celebrate well conducted trials and promote effective treatments but we will also speak up about and challenge interventions that might cause more harm than good. CSO welcomes engagement from all stakeholdersespecially patient advocacy groups. CSO will educate and empower the next generation of oncologists to push our field to do better for patients. We will seek to decrease global and regional inequities in access to affordable high-quality care. Improvements in the generation, interpretation, and communication of evidence will help close these gaps and move our field closer to a future in which a patients outcome is not determined by where they live, what they can afford, or the strength of a marketing campaign. The CSO initiative will undoubtedly evolve over time, but our core mission will continue to ensure that cancer care and innovation is focused on outcomes that matter to patients rather than the commercial bottom line.
(23). Georgina V Long et al. Cutaneous melanoma. Lancet 2023;402(10400):P485-502.
Cutaneous melanoma is a malignancy arising from melanocytes of the skin. Incidence rates are rising, particularly in White populations. Cutaneous melanoma is typically driven by exposure to ultraviolet radiation from natural sunlight and indoor tanning, although there are several subtypes that are not related to ultraviolet radiation exposure. Primary melanomas are often darkly pigmented, but can be amelanotic, with diagnosis based on a combination of clinical and histopathological findings. Primary melanoma is treated with wide excision, with margins determined by tumour thickness. Further treatment depends on the disease stage (following histopathological examination and, where appropriate, sentinel lymph node biopsy) and can include surgery, checkpoint immunotherapy, targeted therapy, or radiotherapy. Systemic drug therapies are recommended as an adjunct to surgery in patients with resectable locoregional metastases and are the mainstay of treatment in advanced melanoma. Management of advanced melanoma is complex, particularly in those with cerebral metastasis. Multidisciplinary care is essential. Systemic drug therapies, particularly immune checkpoint inhibitors, have substantially increased melanoma survival following a series of landmark approvals from 2011 onward.
(24). Juan P Fria et al. Efficacy and safety of oral orforglipron in patients with type 2 diabetes: a multicentre, randomised, dose-response, phase 2 study. Lancet 2023;402(10400):P472-483.
Orforglipron, an oral, non-peptide glucagon-like peptide-1 (GLP-1) receptor agonist, is in development for type 2 diabetes and obesity. We assessed the efficacy and safety of orforglipron versus placebo or dulaglutide in participants with type 2 diabetes.
In this 26-week, phase 2, double-blind, randomised, multicentre study, participants were recruited from 45 centres (private clinics, hospitals, and research centers) in the USA, Hungary, Poland, and Slovakia. Adult participants aged 18 years or older with type 2 diabetes treated with diet and exercise, with or without metformin, and with a glycated haemoglobin (HbA1c) of 70105%, and stable BMI of 23 kg/m2 or more, were randomly assigned (5:5:5:5:5:3:3:3:3) via an interactive web-response system to placebo, dulaglutide 15 mg once per week, or orforglipron 3 mg, 12 mg, 24 mg, 36 mg (group 1), 36 mg (group 2), 45 mg (group 1), or 45 mg (group 2) once per day with no food or water restrictions. Two different dose escalation regimens were evaluated for each of the 36 mg and 45 mg cohorts. Participants were masked to the study drug, dulaglutide, and placebo. The primary efficacy outcome The primary efficacy outcome was mean change in HbA1c from baseline with orforglipron versus placebo at week 26. Efficacy was analysed in all randomly assigned participants who received at least one dose of study drug and excluded data after the permanent discontinuation of study drug or initiation of rescue medication. Safety was analysed in all participants who received at least one dose of study treatment. This trial is registered at ClinicalTrials.gov (NCT05048719) and is completed.
Between Sept 15, 2021, and Sept 30, 2022, 569 participants were screened and 383 were enrolled and randomly assigned to a group. 352 (92%) completed the study and 303 (79%) completed 26 weeks of treatment. At baseline, the mean age was 589 years, HbA1c was 81%, BMI was 352 kg/m2, 226 (59%) were men, and 157 (41%) were women. At week 26, mean change in HbA1c with orforglipron was up to 210% (167% placebo adjusted), versus 043% with placebo and 110% with dulaglutide. HbA1c reduction was statistically superior with orforglipron versus placebo (estimated treatment difference 08% to 17%). Change in mean bodyweight at week 26 was up to 101 kg (95% CI 115 to 87; 79 kg placebo adjusted [99 to 59]) with orforglipron versus 22 kg (36 to 07) for placebo and 39 kg (53 to 24) for dulaglutide. The incidence of treatment-emergent adverse events ranged from 618% to 889% in orforglipron-treated participants, compared with 618% with placebo and 560% with dulaglutide. The majority were gastrointestinal events (441% to 704% with orforglipron, 182% with placebo, and 340% with dulaglutide) of mild to moderate severity. Three participants receiving orforglipron and one participant receiving dulaglutide had clinically significant (54 mg/dL [3 mmol/L]) hypoglycaemia and no participants had severe hypoglycaemia. One death occurred in the placebo group and was not related to study treatment.
In this phase 2 trial the novel, oral, non-peptide GLP-1 receptor agonist orforglipron at doses of 12 mg or greater showed significant reductions in HbA1c and bodyweight compared with placebo or dulaglutide. The adverse event profile was similar to other GLP-1 receptor agonists in similar stage of development. Orforglipron might provide an alternative to injectable GLP-1 receptor agonists and oral semaglutide, with the prospect of less burdensome administration to achieve treatment goals in people with type 2 diabetes.
(25). Catherine Le Berre et al. Ulcerative colitis. Lancet 2023;402(10401):P571-584.
Ulcerative colitis is a lifelong inflammatory disease affecting the rectum and colon to a variable extent. In 2023, the prevalence of ulcerative colitis was estimated to be 5 million cases around the world, and the incidence is increasing worldwide. Ulcerative colitis is thought to occur in people with a genetic predisposition following environmental exposures; gut epithelial barrier defects, the microbiota, and a dysregulated immune response are strongly implicated. Patients usually present with bloody diarrhoea, and the diagnosis is based on a combination of clinical, biological, endoscopic, and histological findings. The aim of medical management is, first, to induce a rapid clinical response and normalise biomarkers and, second, to maintain clinical remission and reach endoscopic normalisation to prevent long-term disability. Treatments for inducing remission include 5-aminosalicylic acid drugs and corticosteroids. Maintenance treatments include 5-aminosalicylic acid drugs, thiopurines, biologics (eg, anti-cytokines and anti-integrins), and small molecules (Janus kinase inhibitors and sphingosine-1-phosphate receptor modulators). Although the therapeutic options are expanding, 1020% of patients still require proctocolectomy for medically refractory disease. The keys to breaking through this therapeutic ceiling might be the combination of therapeutics with precision and personalised medicine.
(26). Stephen C Bai et al. A new class of glucose-lowering therapy for type 2 diabetes: the latest development in the incretin arena. Lancet 2023;402(10401):P504-505.
In 2005, the first GLP-1 receptor agonist was launched as a glucose-lowering treatment for people with type 2 diabetes. GLP-1 is a member of the incretin family, a set of hormones that are released from the gastrointestinal tract following the intake of nutrients, and it had been found to enhance insulin secretion in response to hyperglycaemia. 1 GLP-1 receptor agonists reduced the postprandial rise in glucagon seen in people with type 2 diabetes, both in a glucose-dependent manner, meaning that hypoglycaemia was only seen when co-administered with insulin or insulin secretagogues. GLP-1 receptor agonists also cause weight reduction, initially attributed to slowing of gastric emptying and side-effects of nausea and vomiting, but which is mainly due to central satiety. Being a peptide, the administration of GLP-1 receptor agonists necessitated subcutaneous administration, which was initially twice daily with meals. However, various technological advances have led to the most frequently initiated agents being once-weekly preparations and there is also a co-formulation with a permeation enhancer, which allows for once-daily oral administration.
(27). Eileen C Rife. Janus kinase inhibition in juvenile idiopathic arthritis. 2023;402(10401):P508-509.
The introduction of biologics targeting proinflammatory cytokines (eg, tumour necrosis factor [TNF] and interleukin-6) has revolutionised the care of children with juvenile idiopathic arthritis. More recently, small molecule inhibitors of Janus kinases (JAKs) have shown efficacy in treating adult patients with rheumatoid arthritis. Given positive clinical trial results in adults, JAK inhibitors are now being explored for use in children with juvenile idiopathic arthritis. JAK inhibitors work by disrupting intracellular signalling pathways (via the signal transducer and activator of transcription, or JAK-STAT, pathway) downstream of receptors shared by multiple cytokines. Tofacitinib, which is primarily an inhibitor of JAK1 and JAK3, has already shown efficacy in the treatment of polyarticular course juvenile idiopathic arthritis.
(28). Julio Rosenstock et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 2023;402(10401):P529-544.
According to current consensus guidelines for type 2 diabetes management, bodyweight management is as important as attaining glycaemic targets. Retatrutide, a single peptide with agonist activity at the glucose-dependent insulinotropic polypeptide (GIP), GLP-1, and glucagon receptors, showed clinically meaningful glucose-lowering and bodyweight-lowering efficacy in a phase 1 study. We aimed to examine the efficacy and safety of retatrutide in people with type 2 diabetes across a range of doses.
In this randomised, double-blind, double-dummy, placebo-controlled and active comparator-controlled, parallel-group, phase 2 trial, participants were recruited from 42 research and health-care centres in the USA. Adults aged 1875 years with type 2 diabetes, glycated haemoglobin (HbA1c) of 70105% (530913 mmol/mol), and BMI of 2550 kg/m2 were eligible for enrolment. Eligible participants were treated with diet and exercise alone or with a stable dose of metformin (≥1000 mg once daily) for at least 3 months before the screening visit. Participants were randomly assigned (2:2:2:1:1:1:1:2) using an interactive web-response system, with stratification for baseline HbA1c and BMI, to receive once-weekly injections of placebo, 15 mg dulaglutide, or retatrutide maintenance doses of 05 mg, 4 mg (starting dose 2 mg), 4 mg (no escalation), 8 mg (starting dose 2 mg), 8 mg (starting dose 4 mg), or 12 mg (starting dose 2 mg). Participants, study site personnel, and investigators were masked to treatment allocation until after study end. The primary endpoint was change in HbA1c from baseline to 24 weeks, and secondary endpoints included change in HbA1c and bodyweight at 36 weeks. Efficacy was analysed in all randomly assigned, except inadvertently enrolled, participants, and safety was assessed in all participants who received at least one dose of study treatment. The study is registered at ClinicalTrials.gov, NCT04867785.
Between May 13, 2021, and June 13, 2022, 281 participants (mean age 562 years [SD 97], mean duration of diabetes 81 years [70], 156 [56%] female, and 235 [84%] White) were randomly assigned and included in the safety analysis (45 in the placebo group, 46 in the 15 mg dulaglutide group, and 47 in the retatrutide 05 mg group, 23 in the 4 mg escalation group, 24 in the 4 mg group, 26 in the 8 mg slow escalation group, 24 in the 8 mg fast escalation group, and 46 in the 12 mg escalation group). 275 participants were included in the efficacy analyses (one each in the retatrutide 05 mg group, 4 mg escalation group, and 8 mg slow escalation group, and three in the 12 mg escalation group were inadvertently enrolled). 237 (84%) participants completed the study and 222 (79%) completed study treatment. At 24 weeks, least-squares mean changes from baseline in HbA1c with retatrutide were 043% (SE 020; 468 mmol/mol [215]) for the 05 mg group, 139% (014; 1524 mmol/mol [156]) for the 4 mg escalation group, 130% (022; 1420 mmol/mol [244]) for the 4 mg group, 199% (015; 2178 mmol/mol [160]) for the 8 mg slow escalation group, 188% (021; 2052 mmol/mol [234]) for the 8 mg fast escalation group, and 202% (011; 2207 mmol/mol [121]) for the 12 mg escalation group, versus 001% (021; 012 mmol/mol [227]) for the placebo group and 141% (012; 1540 mmol/mol [129]) for the 15 mg dulaglutide group. HbA1c reductions with retatrutide were significantly greater (p00001) than placebo in all but the 05 mg group and greater than 15 mg dulaglutide in the 8 mg slow escalation group (p=00019) and 12 mg escalation group (p=00002). Findings were consistent at 36 weeks. Bodyweight decreased dose dependently with retatrutide at 36 weeks by 319% (SE 061) for the 05 mg group, 792% (128) for the 4 mg escalation group, 1037% (156) for the 4 mg group, 1681% (159) for the 8 mg slow escalation group, 1634% (165) for the 8 mg fast escalation group, and 1694% (130) for the 12 mg escalation group, versus 300% (086) with placebo and 202% (072) with 15 mg dulaglutide. For retatrutide doses of 4 mg and greater, decreases in weight were significantly greater than with placebo (p=00017 for the 4 mg escalation group and p00001 for others) and 15 mg dulaglutide (all p00001). Mild-to-moderate gastrointestinal adverse events, including nausea, diarrhoea, vomiting, and constipation, were reported in 67 (35%) of 190 participants in the retatrutide groups (from six [13%] of 47 in the 05 mg group to 12 [50%] of 24 in the 8 mg fast escalation group), six (13%) of 45 participants in the placebo group, and 16 (35%) of 46 participants in the 15 mg dulaglutide group. There were no reports of severe hypoglycaemia and no deaths during the study.
In people with type 2 diabetes, retatrutide showed clinically meaningful improvements in glycaemic control and robust reductions in bodyweight, with a safety profile consistent with GLP-1 receptor agonists and GIP and GLP-1 receptor agonists. These phase 2 data also informed dose selection for the phase 3 programme.
(29). Louise Aronson. Introducing Inside Story. JAMA Intern Med. 2023.
The importance of stories and storytelling in medicine and science should not be underestimated.
While rigorous scientific evidence is central to advancing medicine and health care, the value of stories to comprehend, communicate, contextualize, and translate the evidence into practice and policy is vital.
Anecdotes and narratives help clinicians personalize care, learn from individual perspectives and experiences, and embrace the fallible yet extraordinary humanity of all we do in medicine.
Moreover, storytelling is key to turning abstract statistical or scientific evidence into real-world examples to help decision-making by policy makers and patients alike. Thus, we are introducing a new section, entitled Inside Story, to recognize storytelling as aperhaps even thefundamental mode of human learning and communication.
Inside Story shall encompass personal narratives that use story, character, language, imagery, and metaphor to address complex human experiences and illuminate the practice of medicine, experiences of health and illness, and issues in health care.
We acknowledge that not all questions have answers and not all problems have solutions. This allows us to move beyond knowledge to meaning through writing that interprets and tries to make sense of events, knowledge, experiences, relationships, and the self.
For this reason, Inside Story seeks narratives that are more than anecdotes and that, even when focused on the self, move beyond solipsism to universality. Toward that end, we welcome true stories that address the joys and challenges, surprises, and frustrations of practicing internal medicine; confront and challenge the issues of our times, large and small; and powerfully convey experiences of disease, illness, caregiving, and care receiving.
We shall look forward to thoughtful essays and good stories, well told.
(30). Vidisha Master. Chronic Nitrofurantoin-Induced Lung Injury. N Engl J Med. 2023;389:549.
A 95-year-old woman presented to the emergency department with a 4-week history of dyspnea and dry cough. She had not previously reported these symptoms to her doctor. She reported no fevers. For the past 6 months, she had been taking nitrofurantoin daily to prevent recurrent urinary tract infections. Her oxygen saturation was 83% while she was breathing ambient air. On physical examination, inspiratory crackles were noted in the upper lung fields. There was no jugular venous distention or edema. Laboratory studies showed neutrophilic leukocytosis but no eosinophilia or elevations in aminotransferase levels. A chest radiograph was notable for patchy alveolar opacities that were most prominent in the upper lobes (Panel A). A sputum culture and viral respiratory panel were negative. A subsequent computed tomographic image of the chest showed ground-glass and reticular opacities with upper-lobe predominance and moderate bronchiectasis (Panel B). Owing to the patients imaging findings and long-term nitrofurantoin exposure, a diagnosis of chronic nitrofurantoin-induced lung injury was made. In patients with nitrofurantoin-induced lung injury, the radiologic findings are varied and may be diffusely distributed or localized. Treatment with nitrofurantoin was stopped, and a prednisone taper was prescribed. One week later, the patients symptoms had abated. Two months later, repeat imaging showed that the opacities had nearly resolved and the bronchiectasis had stabilized (Panels C and D, respectively).