Ragya Bharadwaj1,*, S. Thilagavathy2
1Infection Control Officer, Kauvery Hospital, Trichy, Tamilnadu, India
2Consultant Microbiologist and Head of Diagnostic Laboratory, Neuberg Ehrlich Laboratory & Kauvery Hospital, Trichy, Tamilnadu, India
Abstract
Mucormycosis is a fungal infection caused by a group of microorganisms belonging to the phylum Glomeromycota. Once classified under rare fungal disease, Mucormycosis is regrouped under emerging pathogens. They are ubiquitous, found mainly in soil and decaying organic matter. The incidence rate of mucormycosis globally varies from 0.005 to 1.7 per million population. In India, prevalence of mucormycosis is estimated as 140 per million population. Also known as Zygomycosis, it mainly infects humans who have uncontrolled diabetes, on chemotherapy or are suffering from a chronic long-term illness. The mode of entry is via inhalation or ingestion of spores. It affects the sinuses, orbit, brain or lungs and can be fatal if left untreated. Most common form is Rhino-orbito-cerebral followed by Pulmonary mucormycosis. COVID-19 associated Mucormycosis (CAM) was mostly isolated during the second wave with mortality rate of almost 31-50%. Unlike the misnomer “Black fungus”, this particular group of fungi have a transparent hyaline ribbon like hyphae. They have predilection to invade blood vessels thereby leading to extensive necrosis (Black eschar). Diagnostic methods include biopsy and fungal staining (KOH mount), which remains the mainstay of laboratory diagnosis. Liposomal amphotericin B is the drug of choice and needs to be initiated early. Prevention of COVID-associated mucormycosis needs to focus on addressing the underlying risk factors aiming for better glycemic control in those with diabetes, appropriate use of systemic corticosteroids and prevention of unnecessary use of antibiotic, antifungal and other immunomodulators. The main aim of this review is to comprehend the microbiology, clinicopathogenesis and effective prevention strategy of mucormycosis especially in this era of COVID-19 pandemic.
Keywords: Mucormycosis, KOH staining, Rhizopus, Hyphae, Amphotericin B, Diabetic ketoacidosis
Background
Pandemic caused due to COVID-19 virus is still present amongst us. Throughout the course of this widespread sickness many secondary infections have come to the fore including opportunistic fungal infections [1]. Mucormycoses are life-threatening fungal infections mostly occurring in hematology, solid organ transplant, or diabetic patients, it may also affect immunocompetent patients following a trauma or burn [2]. Mucormycosis is characterized by host tissue infarction and necrosis resulting from vasculature invasion by hyphae. Most common clinical presentations are rhino-orbito-cerebral and pulmonary. Multicentre studies have reported an increasing incidence probably due to an increase in the at-risk population and improved diagnostic tool [3,4].
A high index of suspicion on the clinician side with the need to send an appropriate sample to the laboratory is a prerequisite. It is not easy to isolate and maintain in the laboratory since their poorly septate hyphae can lose the vital cytoplasm at the least manipulation. The disease warrants efficient training of clinicians and surgeons in order to diagnose effectively and treat efficiently [5].
This review highlights the various aspects of Mucor and its relation to the present virus.
History
In 1855, Kurchenmeister described case of mucormycosis in a patient with neoplastic lung on the basis of its histopathology. Furbringer in 1876, described pulmonary mucormycosis for the first time, which was caused by Absidia (now, Lichtheimia) [6,7].
Platauf coined term ‘Mycosis Mucorina’ and described a well-documented case of systemic infection in 1885, which was popularly called as mucormycosis during those days. In 1943, Gregory and colleagues, in a series of three fatal cases associated with diabetic ketoacidosis, reported more typical findings of advanced rhinocerebral mucormycosis. Many newer species of mucorales have been reported in recent times [8].
Classification
Earlier classified under orders Mucorales and Entomophthorales, they were previously considered subordinate members of phylum Zygomycota and now are elevated to the rank of sub-phylum Mucoromycotina and Entomophthoromycotina under phylum Glomeromycota (Fig. 1) [8].
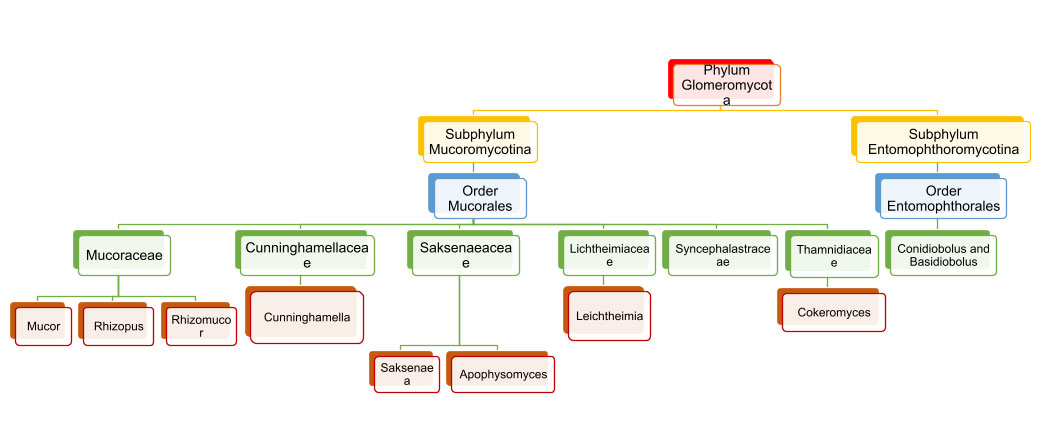 Fig 1. Classification of Phylum Glomeromycota [9].
Fig 1. Classification of Phylum Glomeromycota [9].
A tabulated representation of the common mucor species causing infection is attached herewith. Table 1.[8].
Table 1. Common causative agents of Mucormycosis and Entomophthoramycosis.
Order Mucorales – Mucormycosis
(a) Rhizopus arrhizus (old name R. oryzae)
(b) R. microspores var. rhizopodiformis
(c) Mucor racemosus
(d) Rhizomucor pusillus
(e) Lichtheimia corymbifera (Mycocladus corymbiferus or Absidia corymbifera)
(f) Apophysomyces sp.
(g) Cunninghamella bertholletiae
(h) Saksenaea sp.
(i) Cokeromyces recurvatus
(j) Syncephalastrum racemosum
Order: Entomophthorales – Entomophthoramycosis
(a) Conidiobolomycosis: C. coronatus, C. incongruous and C. lamprauges
(b) Basidiobolomycosis: Basidiobolus ranarum
Mycology
A group of lower fungi with aseptate or sparsely septate hyphae. Hyphae are broad, ribbon like and hyaline (transparent) in appearance. The branching is most commonly seen at 90 degrees. The reproduction is via asexual method by way of sporangiospores. These fungi also reproduce sexually by formation of a single, dark, thick-walled spore called zygospore (earlier known as zygomycetes).
Most of the medically important species are heterothallic and do not form their sexual structures unless two compatible isolates come in contact Fig. 2.
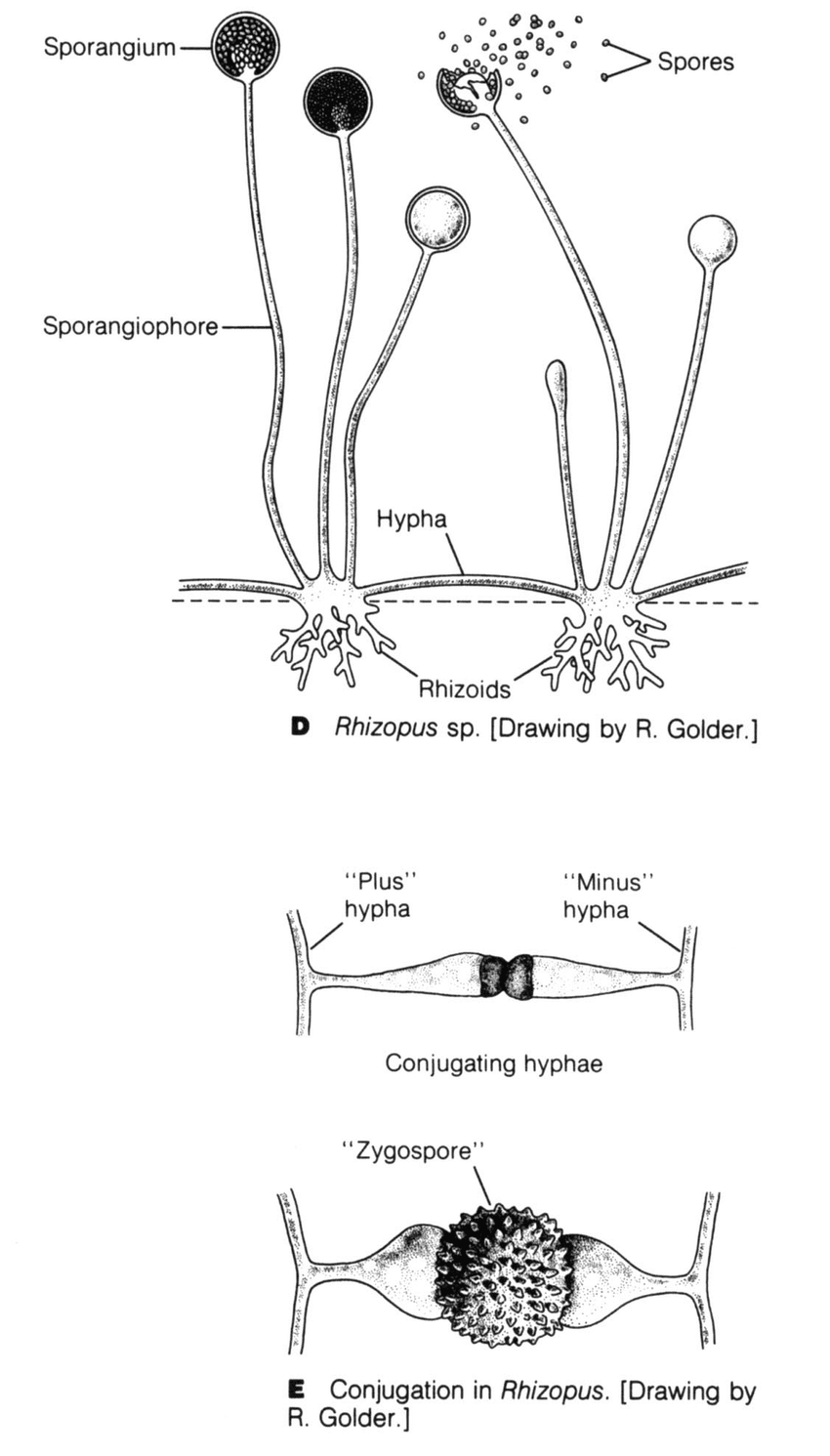 Fig 2. D. Parts of Rhizopus species. E. Sexual reproduction in Rhizopus sp.
Fig 2. D. Parts of Rhizopus species. E. Sexual reproduction in Rhizopus sp.
Nature of spore bearing structure, location of rhizoids, type of zygospores produced and physiological characteristics are used to differentiate species causing mucormycosis.
In some of species like Cokeromyces recurvatus dimorphic conversion to yeast form is observed [8].
The growth on culture in all the cases is rapid, cottony mycelial on SDA. The genus Mucor is characterized by rapidly growing light dull to grey colonies and absence of rhizoids and no distinct columella (Fig 3)
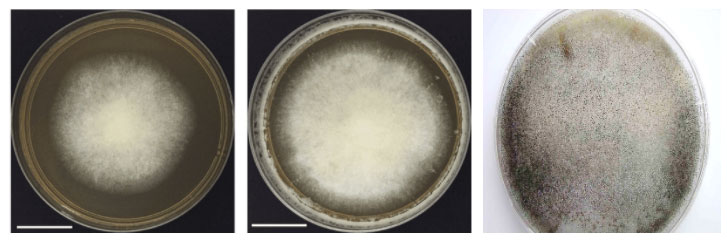 Fig. 3. Growth of Mucor sp.
Fig. 3. Growth of Mucor sp.
On the other hand, Rhizopus has nodal rhizoids directly beneath unbranched sporangiophores and ovoid columella. There is development of sporangiophores from aerial hyphae or stolons with single and weakly branched rhizoids [8].
Etiopathogenesis
Deep tissues mostly get affected by mucorales by means of ingestion or inhalation or percutaneous injection of spores. Prakash et al [16] estimated the difference between Mucorales mean spore count between indoors (0.68-1.12 CFU/m3) and outdoors (0.73-8.60 CFU/ m3).
Patients at risk are with chronic debilitating illnesses like
- Diabetes, especially ketoacidosis
- Long term use of systemic steroids
- Old age
- Neutropenia or any other haematological malignancies
- Organ or stem cell transplantation
- Iron overload
- Any types of trauma to skin
- Broad-spectrum antibiotics
- Intravenous drug abuse
- Prophylactic voriconazole mainly given for aspergillosis [10].
Four major attributes are seen in pathogenesis of Mucormycosis in COVID-19
- Host Defences
- Fungal endothelial interactions
- Role of Iron
- Role of diabetes
Infection in a susceptible host starts with entry of sporangiospore through nasal cavity (most common mode). Spores’ express ligand cot H which interacts with glucose-regulator protein GRP78 receptors on nasal endothelium leading to entry inside blood vessel as seen in Fig. 5. In normal hosts, macrophages prevent initiation of infection by phagocytosis and oxidative killing of spores but in immunocompromised patients’ spores evade the oxidative metabolites and defensins secreted by cells and reach the endothelial lining. Spores and Hyphae interact with EDGF on endothelial cells causing angioinvasion (penetration through endothelial cells lining blood vessels – CRITICAL step) and dissemination into the body [11].
Elaboration of lytic enzymes and proteases along with mycotoxins augment extensive fungal invasion. R. arrhizus spores have the ability to adhere to subendothelial matrix (basement membrane) proteins including laminin and type IV collagen causing extensive damage to the blood vessel. This in turn leads to vessel thrombosis and tissue necrosis (black appearance).
COVID-19 virus increases the ferritin levels thereby increasing intracellular iron (growth factor for mucor). In systemic ketoacidosis there is temporary disruption of transferring to bind iron hence concentration of free iron increases in serum as seen in Fig. 4. The acidic background results in oxidative environment which affects glutathione renovation through GSH/GSSG enzyme cycle and more free iron production by reducing its binding to transferrin [12,13].
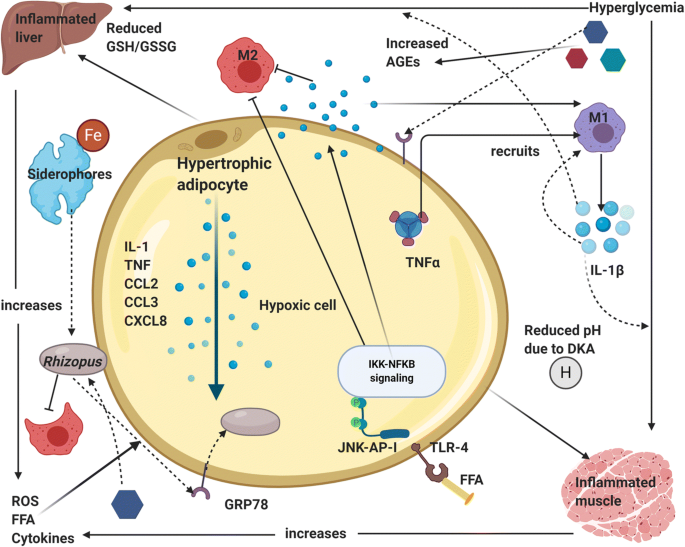 Fig. 4. Immunopathogenesis of comorbidity DM-mucormycosis [14].
Fig. 4. Immunopathogenesis of comorbidity DM-mucormycosis [14].
High levels of glucose cause endoplasmic reticulum (ER) stress and increased release of reactive oxygen species (ROS), free fatty acids (FFA) and cytokines in the liver, muscle, and adipose tissue. There is an upregulation of GRP 78 receptors and their translocation to plasma membrane for binding with hyphal elements receptors cot H. Hypertrophic cells in adipose tissue release pro-inflammatory cytokines like interleukin-1β, TNF-α and chemokines like CCL2, CCL3 and CXCL8 (Fig. 4). M1 macrophages are recruited by the action of TNF-α and its activation releases more pro-inflammatory cytokines (mainly IL-1β) that generate persistent inflammation and the recruitment of more M1 macrophages (cytokine storm). In addition, at the cytoplasm level of tissue cells, FFA are recognized by Toll-like receptors 4 (TLR-4) activating JNK-AP-I and IKK-NFκB signaling [13].
Afterwards, the expression and release of proinflammatory cytokines promote the local inflammatory state. There is tissue infiltration of M1 macrophages in diabetic patients, promoting pro-inflammatory response instead of regulatory response by M2 phages. Advanced glycation end products (AGEs) and reactive oxygen species (ROS) derived from the increased glucose metabolism accumulate in organs and tissues triggering the typical micro- and macrovascular alterations leading to an increased susceptibility to Rhizopus infection (Fig. 5) [12-14].
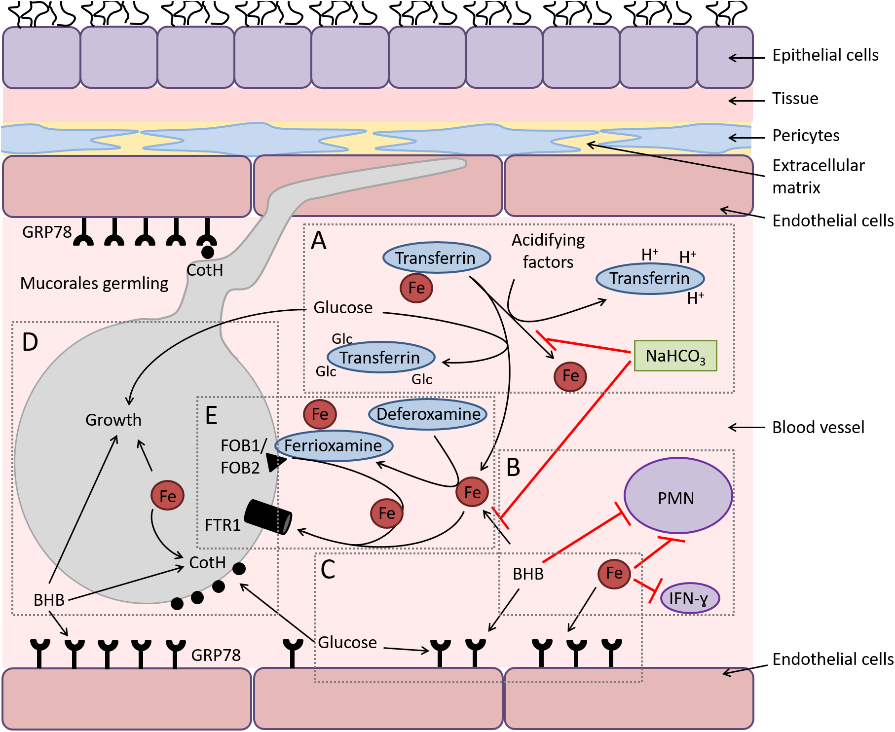
Fig. 5. Diagram depicting the interactions of Mucorales with endothelial cells during hematogenous dissemination/organ seeding and the effect of host factors on these interactions and on the immune response.
Below is a pictographic representation of proposed mechanisms for the establishment of ROCM in DM patients with detailed description attached herewith (Fig 6).
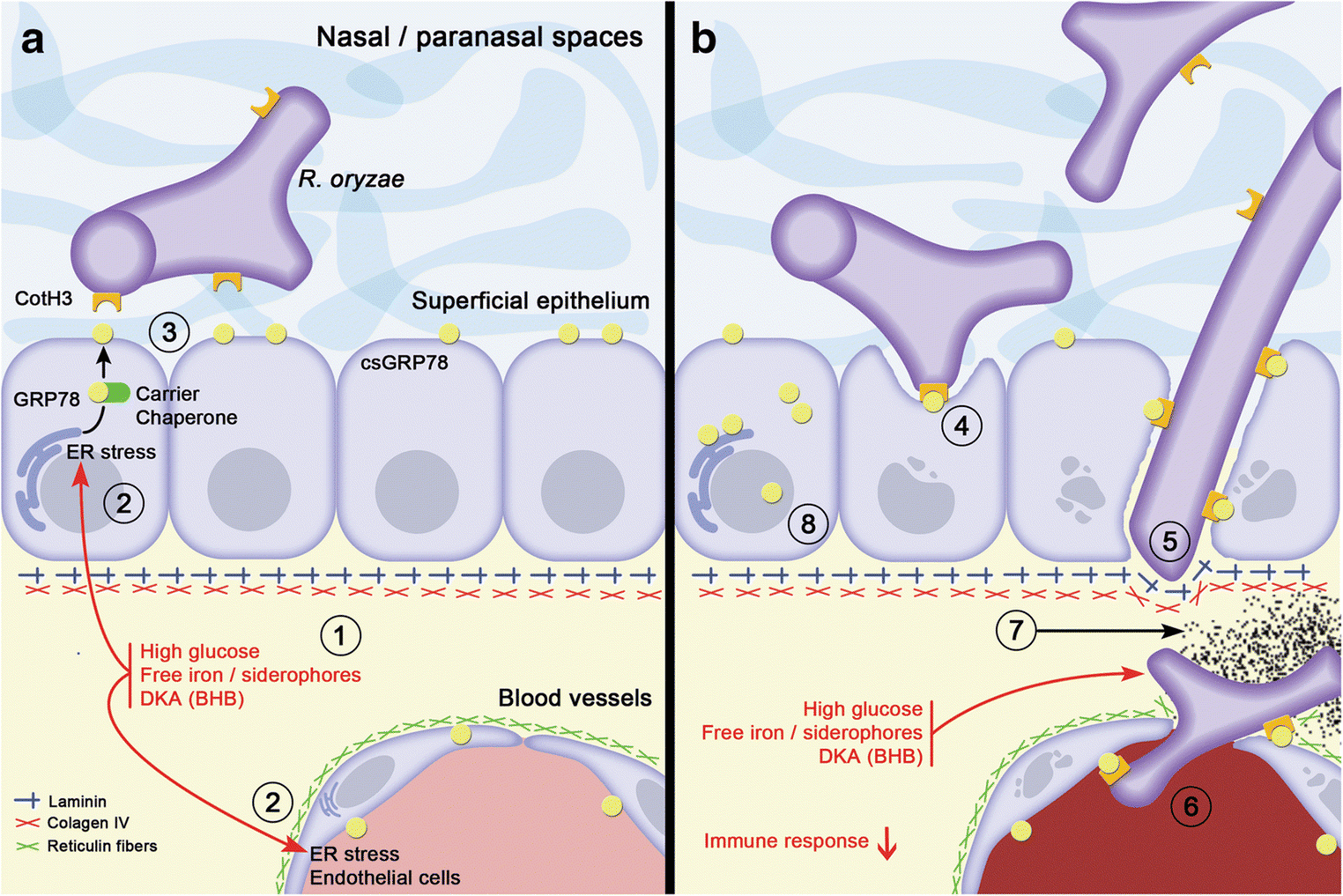
Fig. 6. Proposed mechanisms for the establishment of rhino-orbital-cerebral mucormycosis (ROCM) in diabetic patients by Morales-Franco et al [14].
- Tissular microenvironment in a diabetic patient is altered; there are high levels of glucose, free iron, and ketone bodies (BHB, B-hydroxy butyrate); and these conditions cause stress on the endoplasmic reticulum in the adjacent epithelial/endothelial cells.
- In response to ER stress, GRP78 is overexpressed and relocated in diverse cellular compartments, particularly on the cell surface, carried by several co-chaperone proteins like MTJ-1 and Par-4.
- Once GRP78 is exposed on the cell surface (csGRP78), it favours the possibility of interaction with the hyphae of R. oryzae through the expression of their CotH3 proteins.
- The interaction between the GRP78 and CotH3 proteins promote hyphae that can damage cells and penetrate the epithelium.
- The association of R. oryzae hyphae to laminin and type IV collagen in the basement membrane allows their adherence and entry into the interstitium where the fungi can approach blood vessels. The microenvironmental imbalance offers favourable conditions for fungus growth: energy (high glucose), free iron for its metabolic requirements, as well as a conducive acidic environment generated by ketone bodies.
- Meanwhile, endothelial cells continue to produce GRP78 in all compartments and the hypha can associate with these proteins on the basal side to later interact with GRP78 expressed on the luminal surface of endothelial cells. Once internalized in the lumen of the blood vessels, the fungi consequently induce the extrinsic coagulation pathway activation, consequently cell damage occurs, and all this triggers the formation of thrombus.
- This results in ischemia and sustained hypoxia, which generates infarction of the tissues and its consequent necrosis.
- Finally, the altered microenvironment in diverse tissular compartments of these patients generates GRP78 overexpression, allowing Rhizopus spp. to find this protein in any type of epithelial or endothelial cell, establishing its interaction and continuing its invasive behaviour.
Epidemiology
The incidence of mucormycosis is difficult to estimate since it is not a reportable disease and the risk varies widely in different populations. A review of 929 cases of mucormycosis that were reported between 1940 and 2003 noted that diabetes mellitus was the most common risk factor, found in 36% of cases, followed by hematologic malignancies (17%) and solid organ or hematopoietic cell transplantation (12%) [15]
The estimated prevalence of mucormycosis is around 70 times higher in India than that in global data. Diabetes mellitus is the most common risk factor, followed by haematological malignancy and solid-organ transplant. Patients with post pulmonary tuberculosis and chronic kidney disease are at additional risk of developing mucormycosis in this country.[16]
Clinical Presentation
Mucormycosis is a very rapidly progressive disease thereby may prove fatal if timely diagnosis is not made entailing delay in institution of specific treatment. These clinical types are given below:
(1) Rhinocerebral Mucormycosis
Rhino ocular cerebral Mucormycosis (ROCM) is the most common form, and it is often seen in patients with diabetic ketoacidosis or with uncontrolled diabetes mellitus [17]. A study from India reported that 88% of the patients with ROCM had diabetes mellitus [18]. A similar finding was reported from the United States, where 83% of the patient had diabetes mellitus [19]. Most commonly, it spreads from nasal mucosa to turbinate bones, paranasal sinuses, orbit and palate with eventual extension into brain where massive invasion of blood vessels causes major infarct (Fig 7) [8]. Several species of mucormycetes have been reported as etiological agents but majority of cases are caused by Rhizopus arrhizus.
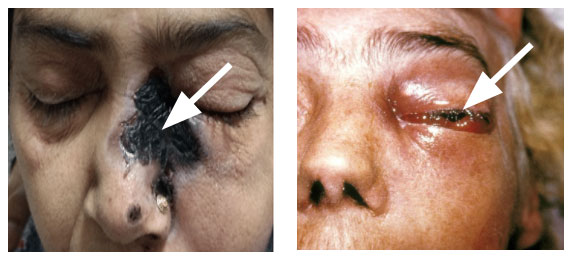 Fig. 7a. Black eschar (photo credit: IP International Journal of Ocular Oncology and Oculoplasty 7(1):49-54). (b) Periorbital swelling (photo credit: Content Providers(s) CDC Dr. Thomas F. Sellers Emory University).
Fig. 7a. Black eschar (photo credit: IP International Journal of Ocular Oncology and Oculoplasty 7(1):49-54). (b) Periorbital swelling (photo credit: Content Providers(s) CDC Dr. Thomas F. Sellers Emory University).
The presenting symptoms include facial pain, headache, lethargy and in advanced cases, loss of vision. The physical examination reveals brownish, bloodstained nasal discharge on the affected side, black eschar on palate due to haemorrhage and tissue necrosis, fixed and dilated pupil and global proptosis and ptosis with dysfunction of cranial nerves, especially fifth and seventh nerves.
There is an extensive and rapid destruction of surrounding tissues. Sometimes, spread may also occur to lungs, gastrointestinal tract, skin and occasionally to other organs. The rhinocerebral mucormycosis is usually fatal as patient dies within a week’s time and invariably diagnosed on autopsy if clinician could not reach tentative diagnosis during life. The signs and symptoms of orbital mucormycosis include chemosis, periorbital cellulitis, ophthalmoplegia,proptosis, ptosis, abrupt visual loss, orbital pain and facial Hypoesthesia.
Clinical Progression
Stage 1: Infection of nasal mucosa and sinuses
Stage 2: Orbital Involvement – Superior orbital fissure syndrome; and Orbital Apex Syndrome
Stage 3: Cerebral Involvement – spread through Opthalmic artery; Superior Orbital Fissure /Cribriform plate.
(2) Pulmonary Mucormycosis
The mucormycetes may present as pulmonary disease through inhalation of sporangiospores. The patients are severely immunocompromised by virtue of an absolute lack of circulating neutrophils, secondary to hematologic malignancy like leukemia, lymphoma, profound immunosuppression or bone marrow transplantation. The lesions may be focal or diffuse and usually uncommon in patients having underlying diabetes mellitus in comparison to rhinocerebral type. The clinical manifestations are nonspecific and may include chest pain, dyspnea and hemoptysis. This entity is suspected when patients have a reverse halo sign on CT of the chest, along with the right clinical findings. Hypersensitivity pneumonitis due to Rhizopus has been reported in Scandinavian Sawmill workers (so-called Wood trimmer’s disease) and in farm workers. [8]
(3) Cutaneous Mucormycosis
The clinical manifestations of cutaneous mucormycosis are varied and range from pustules or vesicles to wounds with wider areas of necrotic zones. In their early stages, lesions resemble ecthyma gangrenosum; cotton-like growth may be seen over surface of tissues, a clinical sign known as ‘hairy pus’. The cutaneous type of mucormycosis can be either primary infection or secondary to the disseminated form.
(4) Gastrointestinal Mucormycosis
The gastrointestinal mucormycosis occurs rarely accounting for ~7% of all cases of mucormycosis, most often involving stomach. It is primarily found among patients suffering from extreme malnutrition and is believed to be acquired by ingesting food contaminated with fungal spores. The agents of gastrointestinal mucormycosis are Lichtheimia corymbifera of Mucorales and Basidiobolus ranarum of Entomophthorales.
(5) Isolated Renal Mucormycosis
The isolated renal mucormycosis is one of the emerging clinical entity, which is an unusual cause of renal infarction and may be fatal if not timely detected. Any of the species of Mucorales may infect the kidneys. The patients usually present with flank pain, fever, and pyuria.
(6) Disseminated Mucormycosis
The mucormycetes may become widely disseminated affecting lungs, kidney, gastrointestinal tract, heart and brain however lungs being the most commonly involved site. The clinical syndromes most frequently reported include pneumonia, stroke, subarachnoid hemorrhage, brain abscess, cellulitis or gangrene of a skin structure.
Complications – Cavernous sinus thrombosis, Multiple cranial nerve palsies, visual loss, Frontal lobe abscess, carotid artery or jugular vein thrombosis [8]
Diagnosis
Mucormycosis diagnosis is challenging. The laboratory diagnosis of mucormycosis is slightly difficult because of rapid and fulminating course of disease and doubtful significance of isolates, which are usually encountered as laboratory contaminants. Therefore, detection of fungus in tissues is supplemented to establish reliability of cultural isolate [8,20]. The detection of circulating antigen such as galactomannan and β-D-1,3-glucan provides no help for mucormycosis diagnosis.
Therefore, samples from the infection site are required to diagnose based on the microscopic detection of typical hyphae and confirmation with culture.
Radiodiagnosis
It has been established that CT and particularly MRI are most helpful in enabling an early detection of orbital, sinus, meningeal, intraparenchymal, cerebral lesions as well as intracranial vascular occlusion, even before clinical signs develop [21,22]. Multiple pulmonary nodules, Pleural effusion and Reverse halo sign occur in the first or second week (Figs. 8 and 9) [20-22].
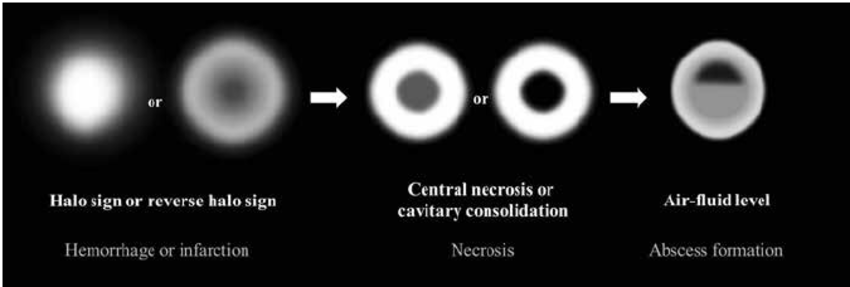
Fig 8. Initially, the halo sign on CT which represents focal consolidation with adjacent ground-glass opacity, develops due to hemorrhage caused by the angioinvasive characteristics. Thereafter, the reversed-halo sign may be seen. After disease progression, internal infarction causes central ground-glass opacity with peripheral consolidation. Necrotic or cavitary consolidation or masses may develop due to necrosis followed by abscess formation with an internal air-fluid line. (photo credit: Choo, Ji & Park, et al, Seoul National University Hospital).
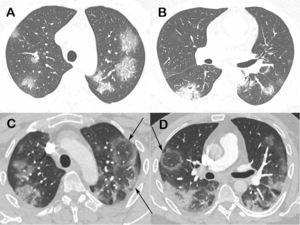
Fig 9. Chest computed tomography images of a 48-year-old man with confirmed COVID-19 pneumonia. Images obtained at the levels of the upper (a) and lower (b) lobes two days after symptom onset show bilateral round and oval ground-glass opacities. Enhanced images (c and d) obtained at the same levels as Fig. 9a and b, three days later show multiple reversed halo signs (arrows) in both lungs.
Nasal endoscopy
Black eschar present in meatus which is painless on removal with no or minimal bleeding.
Histopathology
On histological examination, extensive necrosis is manifested in the affected tissue along with numerous large branching pale-staining, wide, flat non-septal hyphae with branching at right or obtuse angles [21].
Necrotic tissue containing hyphae might be seen with signs of angio-invasion and infarction are seen; in non granulocytopenic conditions, infiltration of the neutrophils and with chronic infection granuloma formation will also be observed. Special stains that highlight fungal wall are Grocott Methanamine Silver (GMS) stain (Fig 10) and Periodic acid Schiff (PAS) stains. PAS gives better visualisation of surrounding tissues [23].
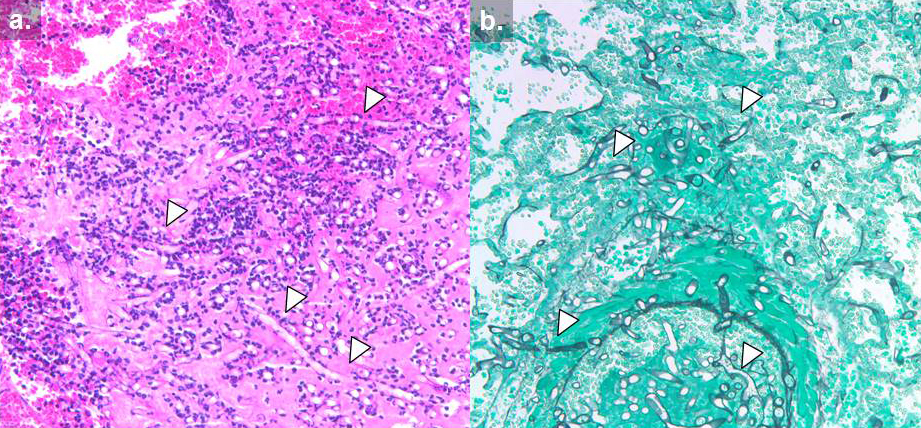
Fig 10. Histology of invasive mucormycosis: (a) hematoxylin and eosin and (b) Grocott’s methenamine silver stain. Arrows indicate hyphal elements. (photo credit: Dr. Russell Lewis. revised by Dr. Sankar Swaminathan.)
Immunohistochemistry
Use monoclonal antibodies against R. arrhizus (recently commercially available) with sensitivity and specificity of 100%
Microbiological diagnosis
(a)Direct Examination with Brightfield Microscopy
microscopic examination of nasal discharge or biopsy material in KOH wet mount shows characteristic broad, non-septate, ribbon-like hyphae with wide-angle or right-angle branching at irregular intervals (Fig 11). Due to absence of cross-walls, fluids from hyphae are free to escape and during handling of biopsy tissues, hyphae collapse and crinkle giving characteristic ribbon-like appearance. Fluorscent brightners such as Blankophor and Calcoflour White together with KOH enhances the characteristic hyphae.
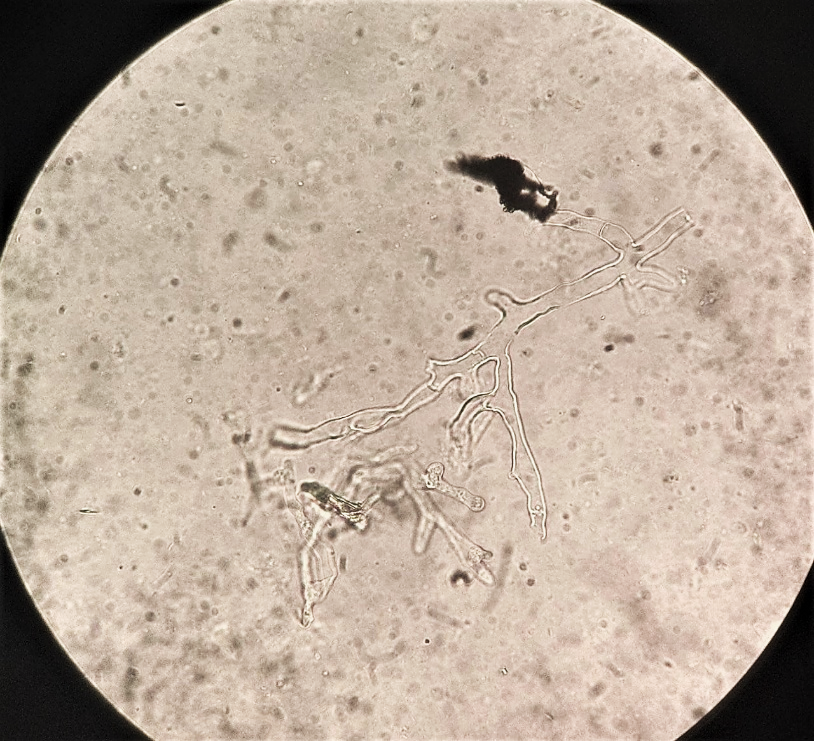
Fig. 11. KOH wet mount shows characteristic broad, non-septate, ribbon-like hyphae with wide-angle or right-angle branching (photo credit: Department of Microbiology Kauvery Hospital Trichy).
(b) Culture
It allows identification to genus and species level. Most medical important Mucorales are thermotolerant and can grow rapidly at 37℃. They grow virtually on any carbohydrate media, colonies appearing within 24-48 h.
Identification is based on colony morphology, microscopic morphology and growth temperature. They grow on SDA with antibiotics at both temperatures i.e. 25°C and 37°C.
Important Note: Specimen should directly be inoculated on culture media without subject to grinding or homogenisation. In about 50% of cases there is no growth despite direct demonstration of the fungi. The reason is that these are sparsely septate fungi and while handling/processing of biopsy entire cytoplasm oozes out losing viability of the organism. Hence enrichment media should also be used for isolation.
The rapidly growing mycelial colonies are white, floccose, dense and have hairy appearance. The mycelia are described as fibrous or cotton-candy growth, which is very vigorous hence some are called as ‘lid-lifters’ as they press upon lid of petri dish from below (Fig. 3) The older colonies are cream or brownish gray. The hyphae are without rhizoids or stolons in Mucor species as compared to Rhizopus.
The rapidly growing mycelial colonies are white, floccose, dense and have hairy appearance. The mycelia are described as fibrous or cotton-candy growth, which is very vigorous hence some are called as ‘lid-lifters’ as they press upon lid of petri dish from below (Fig. 3) The older colonies are cream or brownish gray. The hyphae are without rhizoids or stolons in Mucor species as compared to Rhizopus.
(c) Animal Pathogenicity
Mucormycosis has been tried in experimental animals to demonstrate lesions produced by various Mucorales species. Infection in laboratory has been induced in mice and rabbits to define pathogenicity and mechanism of sporangiospore germination in vivo.
(d) Newer Technologies
MALDTI TOF: Matrix Assisted Laser desorption ionization – time of flight mass spectrometry is commercially available with limited research data on accurate diagnosis. Emerging Molecular methods – ITS Integrated transcribed spacer sequencing (DNA region on fungi) – recommended for species identification [24].
Polymerase chain reaction (PCR): It is recommended for detection of species in tissue samples. Many methods have been developed – Nested, Real time (qPCR), PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) and PCR/ High resolution Melt analysis (HRMA).
Many of these methods have been reportedly successful and perform better on fresh or deep-frozen samples than on paraffin [25].
Most molecular tests target the 18 s ribosomal RNA genes, but also other targets have been investigated (yet to be validated) including mitochondrial gene rnl, cytochrome b gene, mucorales specific cot H gene [26]
A new pan Mucorales real time qPCR commercial kit (Mucorgenius R, Patho Nostics, Maastricht, Netherlands) appeared to be a fast diagnostic test with an overall sensitivity of 75% tested on serial blood samples from culture positive patients. Validation for this test is carried out in house, but only drawback being its inability to identify genus level [26].
Treatment and prophylaxis
Mucormycosis therapy can be divided in four concomitant approaches. These are
- Rapid correction of underlying predisposing factor of the host like diabetic ketoacidosis
- Surgical debridement of necrotizing tissue if this is feasible
- Antifungal therapy
- Consideration of adjunctive therapy such as hyperbaric oxygen.
The combination of surgical debridement and antifungal drugs is required for an ideal treatment of mucormycosis.
It should be preferably done under general anesthesia taking adequate healthy tissue for debridement. Simultaneously management of underlying risk factor, if any, is also essential. There are only few drugs, which are available for treating mucormycosis i.e. intravenous amphotericin B, Posaconazole and isavuconazole. It is observed that start with higher doses of antifungal drugs instead of step-wise increment.
Surgical debridement is the mainstay of therapy for cutaneous mucormycosis and topical amphotericin B is a useful adjunct in concentration of 5 mg/ml, which is applied with a gauge.
In case of posaconazole, it should be kept in mind that the drug becomes effective after a fortnight or so. There is no oral chemoprophylactic agent available for this fungal disease. Cytokines such as interferon-γ and granulocyte-macrophage colony-stimulating factors (GM-CSF) have also been used to treat mucormycosis [8, 26]
Fluconazole, voriconazole, echinocandins (caspofungin, anidulafungin, micafungin) or 5 flurocytosine is not active against mucormycosis (Fig. 12).
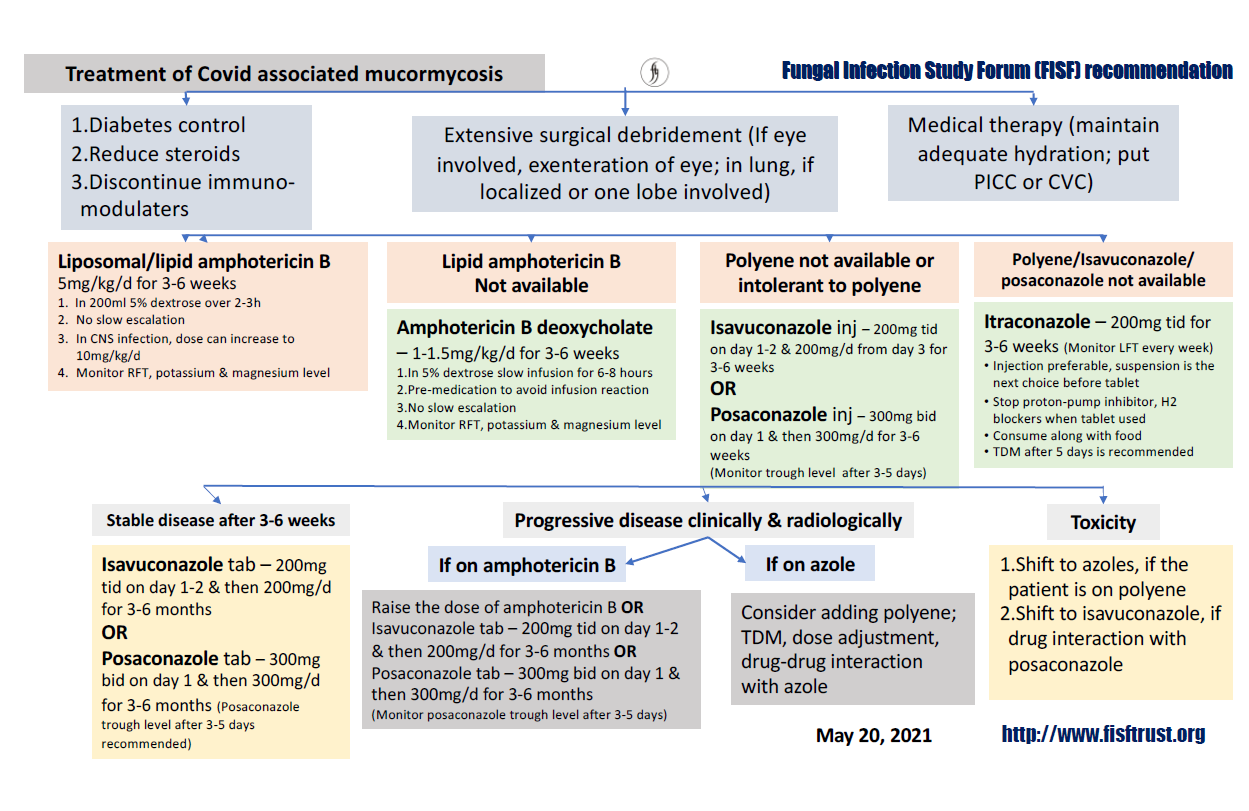
Fig. 12. Treatment of COVID associated Mucormycosis (Fungal Infection Study Forum Recommendation, dated May 20, 2021).
Prevention [27]
- Environmental Intervention
- All walls and roof should be Dust and Mould Free.
- No water logging in any area
- All AHU and AC Vents must be cleaned and filters changed
- No construction work should be done where COVID patients are admitted
- Ensure good ventilation.
- Intervention on Patient and HCW
- Strict adherence to five moments of hand Hygiene
- All patients should wear medical mask when not on Oxygen support
- Patients should maintain good personal hygiene.
- Intervention on Equipment’s and Medical Devices
- Regular survey of humidifiers for any dirt, dust, mould, mildew, stains, or any odd odour
- Only RO /sterile water to be used in oxygen humidifiers. No tap water.
- Oxygen masks and Nasal Cannulas to be cleaned with Soap and Water daily for each patient
- If the Oxygen masks and Nasal Cannulas have to be used for a different patient then it should be sterilized with ETO.
Conclusion
The epidemiology of mucormycosis is evolving. In light of COVID-19 disease, diabetes mellitus still remains the main underlying risk factor for developing this disease. In developed countries most common underlying diseases are haematological malignancies.
An unholy trinity of diabetes, rampant use of corticosteroid in a background of COVID-19 appears to increase mucormycosis. All efforts should be made to maintain optimal glucose and only judicious use of corticosteroids in patients with COVID-19 [27].
Diagnosis of mucormycosis remains challenging. Histopathology, direct examination and culture remain mainstay, although newer tools like molecular methods are improving.
Newer molecular platforms are being investigated and new fungal genetic targets are being explored.
More such needed rapid methods that do not require invasive procedures, such as serology-based point of care hopefully will be evaluated and used in the near future.
References
- Garg D, Muthu V, Sehgal IS. Coronavirus disease (Covid-19) associated mucormycosis (cam): case report and systematic review of literature. Mycopathol. 2021;186:289-98.
- Roden MM, Zaoutis TE, Buchanan WL. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634-53
- Bitar D, Van Cauteren D, Lanternier F. Increasing incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis. 2009;15(9):1395-401.
- Guinea J, Escribano P, Vena A. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS One. 2017;12(6):e0179136.
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012,54,S23-S34.
- Mohammadi R, Nazeri M, Sayedayn SM, Ehteram H. A successful treatment of rhinocerebral mucormycosis due to Rhizopus oryzae. J Res Med Sci. 2014;19(1):72.
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Inf Dis. 2005;41(5):634-53.
- Chander J. Textbook of Medical Mycology. 4th ed. Jaypee brothers’ Medical Publishers; 2018.
- Alvarez E. Spectrum of Zygomycetes species identified in clinically significant specimens in the United States. J. Clin. Microbiol. 2009;47:1650-6.
- Waldorf AR. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser. 1989;47:243-71.
- Liu M, Spellberg B, Phan QT, Fu Y, Lee AS, Edwards JE Jr., et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 120(6):1914-24.
- Waldorf AR, Ruderman N, Diamond RD. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest. 1984;74:150-60.
- Artis WM, Fountain JA, Delcher HK. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes, 1982;31:109-14.
- Morales-Franco B, Nava-Villalba M, Medina-Guerrero EO. Host-Pathogen molecular factors contribute to the pathogenesis of rhizopus spp. in diabetes mellitus. Curr Trop Med Rep. 2021;8, 6-17.
- Roden MM, Zaoutis TE, Buchanan WL. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634.
- Prakash H, Chakrabarti A. Global Epidemiology of Mucormycosis. J Fungi. 2019;5(1):26.
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova, TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634-653.
- Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D’Souza O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J. Ophthalmol. 2003;51:231-6.
- Reed C, Bryant R, Ibrahim AS, Edwards J, Filler SG, Goldberg R, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis. 2008;47:364-71.
- Pilmis B, Alanio A, Lortholary O, Lanternier F. Recent advances in the understanding and management of mucormycosis. Res. 2018;7:1429.
- Nishanth G, Anitha N, Aravindha Babu N, Malathi L. Mucormycosis – a review. Eur J Mol Clin Med. 2020;7.
- Peng M, Meng H, Sun Y, Xiao Y, Zhang H, Lv K, Cai B. Clinical features of pulmonary mucormycosis in patients with different immune status. J Thoracic Dis. 2019;11:2019.
- Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012;54:S55-60.
- Baldin C, Soliman SSM, Jeon HH, Alkhazraji S, Gebremariam T, Gu Y, et al. PCR-based approach targeting mucorales-specific gene family for diagnosis of mucormycosis. J Clin Microbiol. 2018;56(10):e00746-18.
- Dadwal SS, Kontoyiannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn. 2018;18(10):845-54.
- Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):265.
- Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102146.
 Dr. Ragya Bharadwaj
Dr. Ragya Bharadwaj
 Dr. Thilagavathy
Dr. Thilagavathy

