Retrospective analysis of effectiveness of Tocilizumab in the cytokine storm of COVID-19 pneumonia
K. Saisoundharya, G. Dominic Rodriguez*, Ivan A Jones, Stephy George
Department of General Medicine, Kauvery Hospital, Trichy, India
Abstract
Tocilizumab, an Interleukin 6 receptor antagonist, has been used to manage the cytokine storm of COVID 19. There are numerous articles and studies on the effectiveness of Tocilizumab in the cytokine storm of COVID-19 pneumonia. It has been reported to be useful in some studies while some studies report it be not effective.
Background
COVID 19 pneumonia is caused by a novel Corona virus SARS CoV 2 which launched the pandemic of this century, early in the year 2020. COVID 19 infection ranges from a mild flu like illness to severe acute respiratory distress syndrome (SARS) which may cause death. The clinical course of COVID 19 is distinguished by an initial viremic phase, that is observed in the first week of illness, followed by an inflammatory phase remarkable for its associated immune and thrombotic phenomena, from the 2nd week onwards2,3. The viremic phase is characterized by fever, sore throat, myalgia and other constitutional symptoms. In the immune thrombotic phase, by the 7th to 10th day of illness, some patients of COVID 19 enter a phase of cytokine storm when there is rapid deterioration. There is progressive and worsening dyspnea, accompanied by a significant rise in inflammatory cytokines. That leads to hypoxia and respiratory failure due to pulmonary involvement4.
This paper describes our experience on the use of Tocilizumab in managing the cytokine storm of COVID-19 pneumonia and documents our observation of clinical factors and laboratory parameters that predict the outcome post-Tocilizumab administration.
Methods
This retrospective observational study was done to study the effectiveness of Tocilizumab in managing the cytokine storm of COVID 19 pneumonia in patients who were admitted in our hospital from June to December 2020. A total of 60 patients were included in our study.
The aims and objectives of our study were to study the mortality rate by day 28, impact of age, sex, comorbidities, the day of administration of Tocilizumab during the course of the disease, and that of convalescent plasma, and the impact on the outcome. We also analysed the trends of National Early Warning (NEWS 2) score, Absolute Lymphocyte Count (ALC), C Reactive Protein (CRP), Ferritin and D dimer between the survived patients and the patients who succumbed to death post Tocilizumab administration. Statistical analysis was done using SPSS software. Correlational relationship between the two variables was analysed using Pearson’s coefficient. Chi square test, Fischer’s extract test and Student t test were used to find out the association between the categorical variables.
Study area: COVID Intensive care unit, Kauvery medical Centre, a tertiary care hospital, Trichy.
Study population: Patients admitted with COVID 19 who were treated with Tocilizumab from June to December 2020 were included in this study.
Sample size: 60
Study design: Retrospective observational study
Study duration: January 2021 to December 2021
Inclusion Criteria:
- Hospitalized patients with COVID 19 pneumonia diagnosed by positive RT-PCR and evidence of ground glass opacities/ consolidation in CT chest.
- Severe COVID was defined as patients with SpO2 less than 90 and respiratory rate more than 30.
- Cytokine storm was defined as a syndrome clinically characterized by a) acute rapid deterioration in clinical status over a period of 24 to 48 hours which usually occurs in the 6th to 10th day of illness with b) rapid increase in respiratory rate and oxygen requirements accompanied by laboratory evidence of hyper inflammatory syndrome which includes increased levels of one or more of the following
- Ferritin
- CRP
- Lactate Dehydrogenase (LDH)
- D-dimer
Exclusion Criteria:
- Severe allergic reactions to Tocilizumab or other monoclonal antibodies
- Active Tb infection
- Suspected active bacterial or fungal infection
- Treatment with immunosuppressive or immuno modulatory therapy within the past 3 months
- Pregnant and lactating mothers.
- Absolute Neutrophil Count <500
- Platelet count<50000
- AST/ALT>5 times the upper limit of normal
- History of diverticulitis or bowel perforation
Results:
Table 1. Association of age with survival and mortality post Tocilizumab administration
| Age vs Status | |||||||
| Age | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Less than 50 | 9 | 90.00% | 1 | 10.00% | 10 | 16.70% | χ2= 0.044 SIG |
| More than 50 | 28 | 56.00% | 22 | 44.00% | 50 | 83.30% | |
| Total | 37 | 61.70% | 23 | 38.30% | 60 | ||
Among the study population (60 people), 10 (16.7%) were in the age group of age less than 50 and 50 people (83.3%) were in the age group of age more than 50. Among 10 people who were in the age group of less than 50, 9 (90%) survived post Tocilizumab administration and 1 (10%) succumbed to death. Among 50 people who were in the age group more than 50, 28 (56%) survived post Tocilizumab administration and 22 (44%) of them succumbed to death. The mortality rate compared between age group less than 50 with age group more than 50 was statistically significant (p value 0.044). The mortality rate was higher among people with age group more than 50 when compared with age group less than 50.
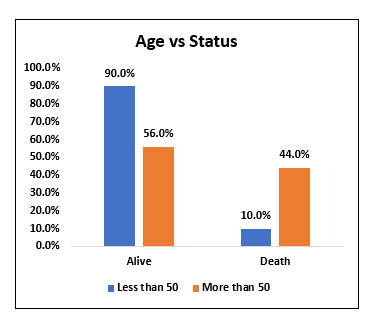
Fig. 1. Bar chart representation of survival and mortality rate post Tocilizumab administration in age group less than 50 and age group more than 50.
Table 2. Association of Gender with Survival and Mortality post Tocilizumab administration
| Gender vs Status | |||||||
| Gender | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Male | 32 | 61.50% | 20 | 38.50% | 52 | 86.70% | χ2= 0.958 NS |
| Female | 5 | 62.50% | 3 | 37.50% | 8 | 13.30% | |
| Total | 37 | 61.70% | 23 | 38.30% | 60 | ||
Among the study population (60), 52 people (86.7%) were males 8(13.3%) were females. Among 52 males who received Tocilizumab, 32(61.5%) of them survived whereas 20 people (38.5%) died. Among 8 females who received Tocilizumab, 5 (62.5%) of them survived and 3(37.5%) of them died. There was no significant difference in mortality when compared between male and female gender.
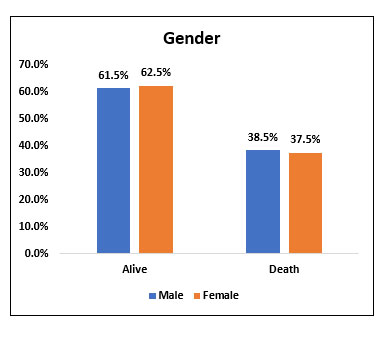
Fig. 2. Bar chart representing survival and mortality post Tocilizumab administration in Male and Female gender.
Table 3. Association of Diabetes with Survival and Mortality post Tocilizumab administration
| DM vs Status | |||||||
| DM | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Yes | 26 | 65.00% | 14 | 35.00% | 40 | 66.70% | χ2= 0.453 NS |
| No | 11 | 55.00% | 9 | 45.00% | 20 | 33.30% | |
| Total | 37 | 61.70% | 23 | 38.30% | 60 | ||
Among the study population (60), 40 people (66.7%) had Diabetes and 20 people (33.3%) did not have history of Diabetes. Among 40 people who were diabetic, 26 people (65%) survived and 14(35%) people succumbed to death.
Among 20 people who were not diabetic, 11 people (55%) survived and 9 people (45%) succumbed to death. There was no statistically significant difference in mortality when comparing diabetic people who received Tocilizumab with people who did not have Diabetes and received Tocilizumab.
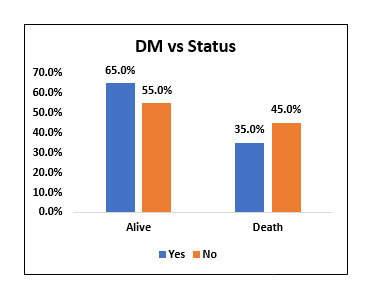
Fig. 3. Bar chart representing survival and mortality post Tocilizumab in Diabetic vs Non diabetic patients.
Table 4. Association of Systemic Hypertension with Survival and Mortality post Tocilizumab administration.
| SHT vs Status | |||||||
| SHT | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Yes | 15 | 55.60% | 12 | 44.40% | 27 | 45.00% | χ2= 0.379 NS |
| No | 22 | 66.70% | 11 | 33.30% | 33 | 55.00% | |
| Total | 37 | 61.70% | 23 | 38.30% | 60 | ||
Among the study population (60), 27 people (45%) had systemic hypertension and 33 people (33%) did not have hypertension. Among 27 people with Hypertension, 15 people (55.6%) survived and 12 people (44.4%) died, whereas among 33 people, who did not have hypertension, 22 people (66.7%) survived and 11 people (33.3%) succumbed to death.
There was no statistically significant difference in mortality when comparing people with Hypertension with people who did not have Hypertension.
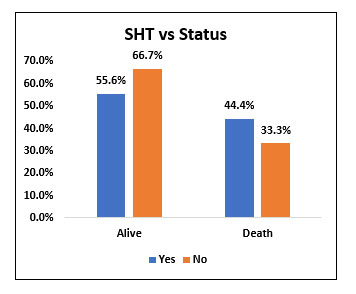
Fig. 4. Bar chart representing the survival and mortality post Tocilizumab in Hypertensive and non hypertensive patients.
Table 5. Association of Coronary Artery Disease with Survival and Mortality post Tocilizumab administration
| CAD vs Status | |||||||
| CAD | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Yes | 2 | 33.30% | 4 | 66.70% | 6 | 10.00% | χ2= 0.132 NS |
| No | 35 | 64.80% | 19 | 35.20% | 54 | 90.00% | |
| Total | 37 | 61.70% | 23 | 38.30% | 60 | ||
Among study population (60), 6 people had H/O coronary artery disease. The mortality rate was 66.7% among people with coronary artery disease and 35.2% among people without coronary artery disease. However, it was not statistically significant.
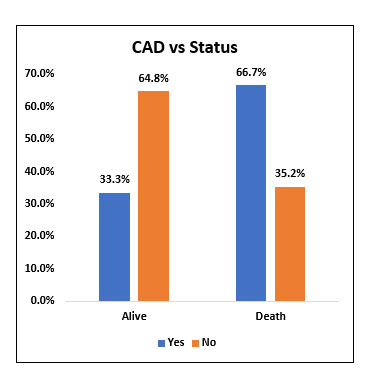
Fig. 5. Bar chart representing the survival and mortality post Tocilizumab in patients with and without Coronary Artery Disease (CAD).
Table 6. Association of Cancer with Survival and Mortality post Tocilizumab administration
| CANCER vs Status | |||||||
| CANCER | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Yes | 0 | 0.00% | 2 | 100.00% | 2 | 3.30% | χ2= 0.068 NS |
| No | 37 | 63.80% | 21 | 36.20% | 58 | 96.70% | |
| Total | 37 | 61.70% | 23 | 38.30% | 60 | ||
Among the study population (60), 2 patients with cancer received Tocilizumab and both of them succumbed to death. However, there was no statistically significant difference in mortality between the two groups.
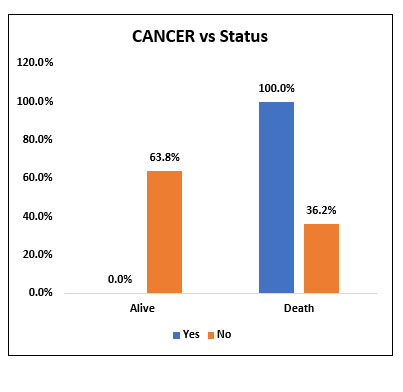
Fig. 6. Bar chart representing the survival and mortality post Tocilizumab in patients with and without Cancer.
Table 7. Association of Chronic Kidney Disease with Survival and Mortality post Tocilizumab administration.
| CKD vs Status | |||||||
| CKD | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Yes | 0 | 0.00% | 1 | 100.00% | 1 | 1.70% | χ2= 0.201 NS |
| No | 37 | 62.70% | 22 | 37.30% | 59 | 98.30% | |
| Total | 37 | 61.70% | 23 | 38.30% | 60 | ||
Among the study population (60), 1 patient with chronic kidney disease received Tocilizumab and he succumbed to death. The significance of effectiveness of Tocilizumab in patients with chronic kidney disease could not be well studied.
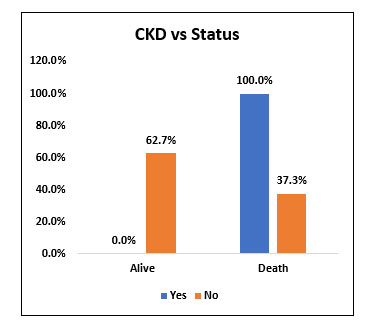
Fig. 7. Bar chart representing the survival and mortality post Tocilizumab in patients with and without Chronic Kidney Disease (CKD).
Table 8. Association of Convalescent plasma transfusion with Survival and Mortality post Tocilizumab administration.
| CPT vs Status | |||||||
| CPT | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Yes | 28 | 70.00% | 12 | 30.00% | 40 | 66.70% | χ2= 0.060 NS |
| No | 9 | 45.00% | 11 | 55.00% | 20 | 33.30% | |
| Total | 37 | 61.70% | 23 | 38.30% | 60 | ||
Among the study population (60), 40 people (66.7%) received convalescent plasma transfusion along with Tocilizumab and 20 people (33.3%) did not receive convalescent plasma transfusion. Among 40 people who received convalescent plasma transfusion, 28 people (70%) survived and 12 people (30%) died. Among 20 people who did not receive convalescent plasma, 9 (45%) people survived and 11 people (55%) succumbed to death. Convalescent plasma transfusion did not prove statistically significant decrease in mortality when compared with patients who didn’t receive convalescent plasma transfusion.
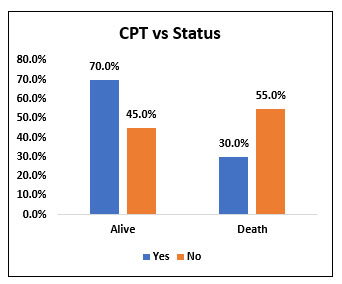
Fig. 8. Bar chart representing the survival and mortality in post Tocilizumab patients who received Convalescent plasma transfusion and who didn’t receive Convalescent plasma transfusion.
Table 9. Association of Day of illness with Survival and Mortality post Tocilizumab administration
| DOI ON TOCI * Status | |||||||
| DOI ON TOCI | Status | Total | % | P - Value | |||
| Alive | % | Death | % | ||||
| Less than 14 | 35 | 67.30% | 17 | 32.70% | 52 | 87% | χ2= 0.022 SIG |
| More than 14 | 2 | 25.00% | 6 | 75.00% | 8 | 13% | |
| Over All | 37 | 23 | 60 | ||||
Among study population (60), 52 people (87%) received Tocilizumab in less than 14 days of illness and 8 people (13%) received Tocilizumab after 14 days of illness. Among 52 people who received Tocilizumab less than 14 days of illness, 35 people (67.3%) survived and 17 people (32.7%) died. Among 8 people who received Tocilizumab more than 14 days of illness, 2 people (25%) survived and 6 people (75%) died. There was statistically significant reduction in mortality when Tocilizumab was given within 14 days of illness when compared with people who received Tocilizumab after 14 days of illness.
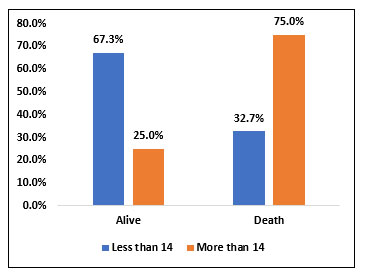
Fig 9. Bar chart representing the survival and mortality rate in patients who received Tocilizumab within 14 days of illness and those who received after 14 days of illness.
Table 12. Analysis of mean Absolute Lymphocyte Count before Tocilizumab, 2 Days, 1 week, 2 week, 28 days post Tocilizumab administration in the survivor and non survivor groups.
| ALC BEFORE TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 37 | 721.62 | 477.339 | χ2= 0.042 SIG |
| Nonsurvivor | 23 | 534.78 | 210.214 | |
| ALC 2 DAYS AFTER TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 37 | 805.41 | 567.62 | χ2= 0.044 SIG |
| Nonsurvivor | 19 | 552.63 | 345.396 | |
| ALC 1WEEK AFTER TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 37 | 994.59 | 814.434 | χ2= 0.001 SIG |
| Nonsurvivor | 14 | 490.71 | 246.466 | |
| ALC 2WEEKS AFTER TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 21 | 1266.67 | 1073.468 | χ2= 0.013 SIG |
| Nonsurvivor | 9 | 522.22 | 454.911 | |
| ALC 28 DAYS AFTER TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 4 | 950 | 465.475 | χ2= 0.099 NS |
| Nonsurvivor | 2 | 400 | 0 | |
In our study population (60), 37 patients survived post Tocilizumab administration and 23 patients succumbed to death post Tocilizumab. We studied the absolute lymphocyte count (ALC) before Tocilizumab, 2 days, 1 week; 2 weeks and 28 days post Tocilizumab in the survived and non survived group.
We compared the mean ALC value before Tocilizumab between the survivor group and the non survivor group and found that the Absolute Lymphocyte count prior to Tocilizumab was significantly higher in the survivor group when compared to the non survivor group. (P value=0.042)
When comparing the mean ALC value 2 days post Tocilizumab administration between the survived group and non survived group we found that the NEWS 2 score was significantly high in the survived group compared to the non survived group (P value=0.044)
When comparing the mean ALC value 1 week post Tocilizumab between the survived group and the non survived group we found that ALC value was persistently high in the survived group compared to the non survived group. (P value=0.001)
When comparing the mean ALC value 2 weeks post Tocilizumab between the survivor group and the non survivor group, we found that the ALC value was persistently high in the survivor group compared to the non survivor group. (P value=0.013)
When comparing the mean ALC value 28 days post Tocilizumab between the survivor group and the non survivor group, we found that the ALC value was high in the survivor group compared to the non survivor group but it was not statistically significant. (P value=0.099).
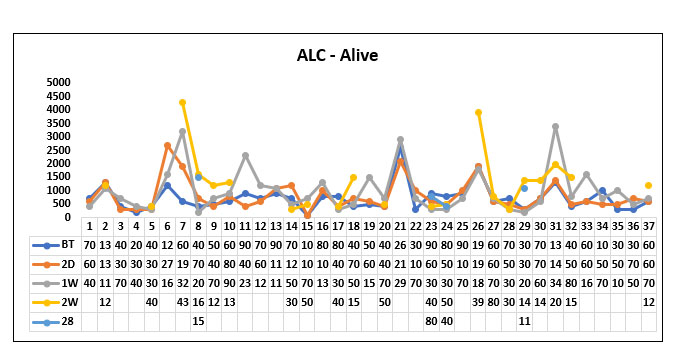
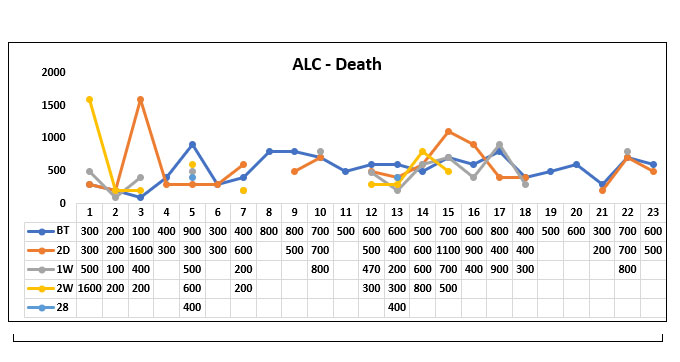
Fig. 11. Line diagram showing the trends of Absolute lymphocyte count (ALC) in the survivor group and non survivor group.
The above tables show the trends in the ALC value before Tocilizumab and 2 days, 1week, 2 week and 28 days post Tocilizumab administration in the group who survived post Tocilizumab administration and the group, who succumbed to death.
Table 12. Analysis of mean C Reactive Protein before Tocilizumab, 2 Days, 1 week, 2-week, 28 days post Tocilizumab administration in the survivor and non-survivor groups.
| CRP BEFORE TOCILIZUMAB | ||||
| Group | N | Mean | Std. Deviation | P - Value |
| Survivor | 37 | 100.93 | 59.851 | χ2= 0.416 NS |
| Nonsurvivor | 23 | 114.85 | 66.137 | |
| CRP 2DAYS AFTER TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 37 | 41.67 | 38.868 | χ2= 0.491 NS |
| Nonsurvivor | 19 | 52.48 | 61.448 | |
| CRP 1WEEK AFTER TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 37 | 9.31 | 13.924 | χ2= 0.522 NS |
| Nonsurvivor | 14 | 6.78 | 7.151 | |
| CRP 2WEEKS AFTER TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 21 | 3.7 | 8.127 | χ2= 0.507 NS |
| Nonsurvivor | 9 | 6.19 | 11.765 | |
| CRP 28 DAYS AFTER TOCILIZUMAB | ||||
| N | Mean | Std. Deviation | P - Value | |
| Survivor | 4 | 2.88 | 3.557 | χ2= 0.191 NS |
| Nonsurvivor | 2 | 57.1 | 79.337 |
In our study population (60), 37 patients survived post Tocilizumab administration and 23 patients succumbed to death post Tocilizumab. We studied the CRP value before Tocilizumab, 2 days, 1 week; 2 weeks and 28 days post Tocilizumab in the survivor and non survivor group.
We compared the mean CRP value before Tocilizumab between the survivor group and the non survivor group and found that the CRP value prior to Tocilizumab was higher in the non survivor group when compared to the survivor group but it was not statistically significant (P value=0.416)
When comparing the mean CRP value 2 days post Tocilizumab administration between the survivor group and the non survivor group we found that the CRP value significantly decreased in both the groups but there was no significant difference between the survivor group and the non survivor group. (P value=0.0491)
When comparing the mean CRP value 1 week post Tocilizumab between the survived group and the non survivor group we found that CRP value was almost near normal in both the groups (P value=0.522)
When comparing the mean CRP value 2 weeks post Tocilizumab between the survivor group and the non survivor group, we found that the CRP value was normal in both the groups (P value=0.507)
When comparing the mean CRP value 28 days post Tocilizumab between the survivor group and the non survivor group, we found that the CRP value in the non survivor group again increased but it was not statistically significant. (P value=0.191).
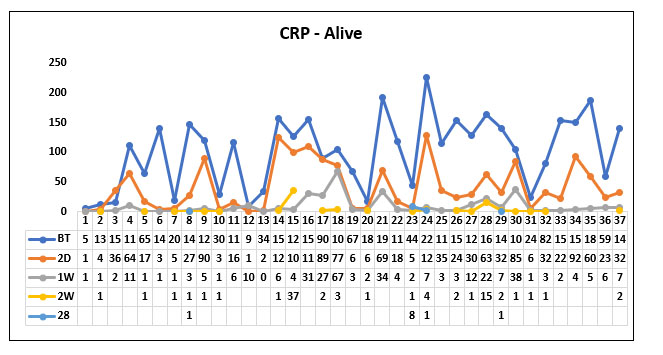
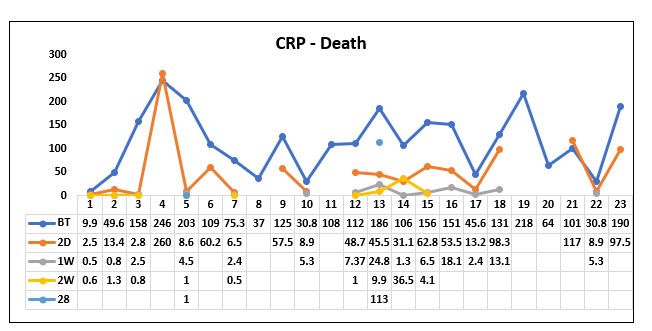
Fig 12. Line diagram showing the trends of C Reactive Protein (CRP) in the survivor and non survivor group.
The above tables show the trends in the CRP value before Tocilizumab and 2 days, 1week, 2 week and 28 days post Tocilizumab administration in the group which survived post Tocilizumab administration and the group which succumbed to death.
Table 13. Analysis of Ferritin before Tocilizumab, 2 Days, 1 week, 2 week, 28 days post Tocilizumab administration in the survivor and non survivor groups.
| FERRIN BEFORE TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 37 | 755.57 | 449.8 | χ2= 0.117 NS |
| Nonsurvivor | 23 | 582.65 | 333.835 | |
| FERRITIN 2 DAYS AFTER TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 37 | 757.2 | 391.353 | χ2= 0.203 NS |
| Nonsurvivor | 19 | 610.58 | 405.058 | |
| FERRITIN 1 WEEK AFTER TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 37 | 632.36 | 387.902 | χ2= 0.340 NS |
| Nonsurvivor | 14 | 517.84 | 352.953 | |
| FERRITIN 2 WEEKS AFTER TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 20 | 560.71 | 300.848 | χ2= 0.269 NS |
| Nonsurvivor | 9 | 726.92 | 489.513 | |
| FERRITIN 28 DAYS AFTER TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 4 | 342.41 | 88.974 | χ2= 0.395 NS |
| Nonsurvivor | 2 | 662.5 | 760.14 |
When comparing the ferritin value before Tocilizumab, 1 week, 2 weeks, 28 days after Tocilizumab between the survived and the death groups we could not find any statistically significant difference between the two groups.
But we could observe that the trend of the ferritin values in the survived group was gradually decreasing post Tocilizumab administration whereas the ferritin values remained in the higher levels in patients who died post Tocilizumab administration. However, it was not significant.
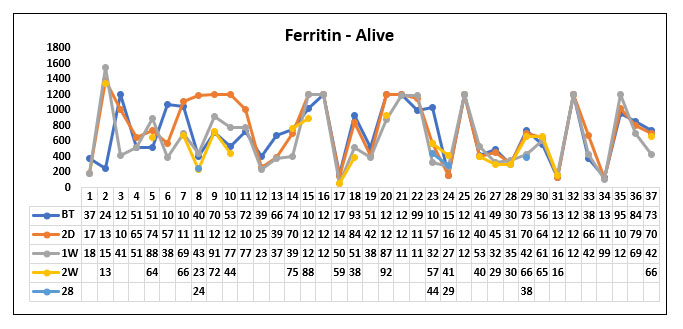
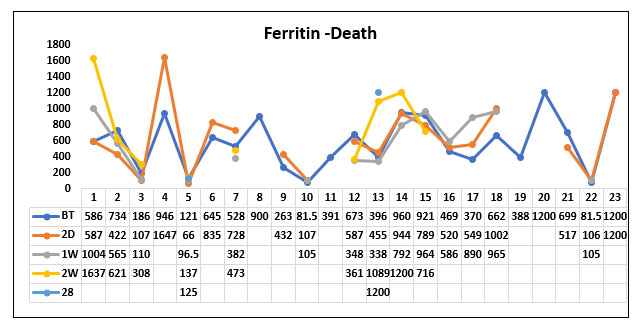
Fig. 13. Line diagram showing the trend of Ferritin in the survivor and non survivor group.
The above tables show the trends in the Ferritin value before Tocilizumab and 2 days, 1week, 2 week and 28 days post Tocilizumab administration in the group which survived post Tocilizumab administration and the group, which succumbed to death.
Table 14. Analysis of D-dimer before Tocilizumab, 2 Days, 1 week, 2 week, 28 days post Tocilizumab administration in the survivor and non-survivor groups.
| D DIMER BEFORE TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 37 | 1756.85 | 2380.206 | χ2= 0.497 NS |
| Nonsurvivor | 23 | 2206.81 | 2626.117 | |
| D DIMER 2 DAYS AFTER TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 37 | 1580.47 | 2102.67 | χ2= 0.200 NS |
| Nonsurvivor | 19 | 2438.47 | 2762.242 | |
| D DIMER 1 WEEK AFTER TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 37 | 1174.27 | 1731.431 | χ2= 0.312 NS |
| Nonsurvivor | 14 | 1651.09 | 1369.253 | |
| D DIMER 2 WEEKS AFTER TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 21 | 740.01 | 579.289 | χ2= 0.035 SIG |
| Nonsurvivor | 9 | 1385.02 | 1015.808 | |
| D DI 28 DAYS AFTER TOCILIZUMAB | ||||
| GROUP | N | Mean | Std. Deviation | P - Value |
| Survivor | 4 | 578.45 | 390.494 | χ2= 0.021 SIG |
| Nonsurvivor | 2 | 1995 | 579.828 |
When comparing the mean D dimer values before Tocilizumab between the survived group and the non survived group, we found that D dimer values were high in both the groups with no significant difference (P value=0.497)
When comparing the D dimer values 2 days and 1 week after Tocilizumab between the survived group and the non survived group, we found that the D dimer value was gradually decreasing in the survived group compared to the non survived group but was not statistically significant. (P value=0.2 and 0.312 respectively)
When comparing the D-dimer values 2-week post Tocilizumab in the survived group and the non survived group, we found that there was a statistically significant decrease in D dimer values in the survived group compared with the non survived group. (P value=0.035). The change persisted even beyond 2 weeks.
When comparing the D-dimer values 28 days post Tocilizumab in the survived group and the non survived group, we found that D dimer values persistently remained high in the non survivor group compared with the survivor group. (P value=0.021).
D-dimer(survivor group)
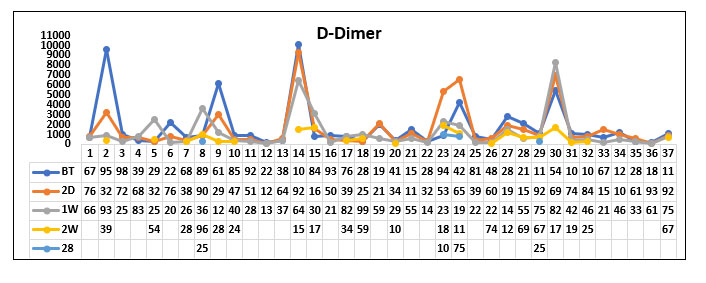
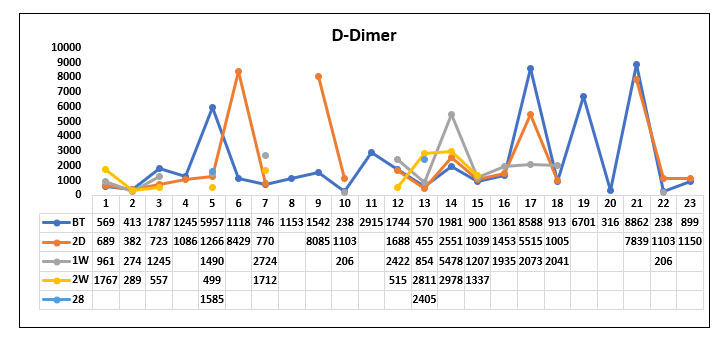
Fig. 14. Line diagram showing the Trend of D dimer in survivor group and non survivor group.
The above tables show the trends in the D-dimer value before Tocilizumab and 2 days, 1week, 2 week and 28 days post Tocilizumab administration in the group which survived post Tocilizumab administration and the group which succumbed to death.
Discussion.
Among the study population (60 people), the clinical outcome in terms of survival and mortality were studied. Among 60 people who received Tocilizumab, 61.7% people (37) people survived and 38.3% people (23) died. Mortality was higher among people with age group more than 50 when compared with people aged less than 50 years. Moreover, the difference in mortality among people with two age groups was statistically significant. The results of our study was consistent with the study conducted by Onder G et al on the Case fatality rate and characteristics of patients dying in relation to COVID 19 in Italy. This study also observed that older age has been associated with poorer COVID 19 outcomes 5
When comparing the mortality rate between the male and female gender, there was no significant difference in mortality with 38.5% and 37.5% mortality in male and female gender respectively. In general, females have a higher macrophage and neutrophil activity as well as antibody production and response. Furthermore in vivo studies of the ACE 2 showed higher expression in the kidneys of males than females6. This explains why the mortality in COVID 19 is higher in male patients compared to female patients. In our study also, the mortality rate was higher in male gender compared to female gender but was not statistically significant.
People with Diabetes who received Tocilizumab had lower mortality (35%) when compared with people who did not have Diabetes and received Tocilizumab (45%), but was not statistically significant. A study conducted by Miftode et al stated that patients with Diabetes diagnosed with SARS – CoV-2 infections were more predisposed to immunological and organ dysfunction that ultimately resulted in death whereas treatment with monoclonal anti IL 6 antibodies was more effective in diabetic patients than nondiabetic patients. (P value -0.05)7. The effectiveness of Tocilizumab was significant in both the studied groups, but diabetic patients responded better to Tocilizumab than non-diabetics (76.7% vs 35%; p vavalue-0.001). Our study didn’t demonstrate statistically significant effectiveness of Tocilizumab in diabetic patients but showed considerable low mortality in diabetics compared to non-diabetics consistent with this study.
Mortality rate was higher among people with Systemic hypertension who received Tocilizumab (44%) when compared with people who did not have hypertension and received Tocilizumab. Yet, the mortality rate between the two groups was not statistically significant. Among our study population, only 10% of them had coronary artery disease. When comparing the two groups, the mortality rate was higher in the group with CAD when compared with the group with no CAD. However, the results were not statistically significant. In our study, out of 60, 2 people had cancer. Both the people with cancer who received Tocilizumab succumbed to death. Since there were only 2 people with cancer, we couldn’t correctly predict the outcome of Tocilizumab administration in patients with cancer. Similarly, there was only one person with CKD who received Tocilizumab and he succumbed to death. So, the outcome of Tocilizumab administration in persons with CKD also could not be well predicted. In a study conducted by Tsai et al on the impact of Tocilizumab administration on mortality in severe COVID 19, advanced age, coronary artery disease and malignancy were significantly associated with mortality in patients with COVID 19 who received Tocilizumab8.This is consistent with our study results with 66.7% mortality in patients with coronary artery disease, and 100% mortality in patients with cancer and CKD. However, the results were not statistically significant and number of patients with the comorbidities of CAD, Cancer and CKD are too low in our study population to be statistically analyzed.
We also studied the association between the day of illness on which the patient received Tocilizumab and the mortality. We found that the mortality rate was lower in people who received Tocilizumab within 14 days of illness when compared with people who received Tocilizumab after 14 days of illness. Moreover, the difference in mortality rate was statistically significant. The results of our study was controversial with the study conducted by Moreno et al in Spain, which evaluated whether timing matters in the administration of Tocilizumab. There was a statistically significant difference in 90-day mortality in the subgroup, which received Tocilizumab within 10 days of onset of symptoms when compared with that of the group that received the drug after 10 days from the onset of illness (18.6% vs 5%; p value 0.048)9
In our study 66.7% of population received Convalescent plasma transfusion along with Tocilizumab. Among them 70% survived (P value=0.06). The results were near significant. The results of our study were at variance with the results of the study conducted by Khamis et al on the role of convalescent plasma and Tocilizumab administration in the management of Covid 19 infection. As per this study, all inflammatory markers showed a gradual decline over time and the ventilator parameters improved in the group which received both Tocilizumab and Convalescent plasma and the mortality rate was lower in the group which received both Tocilizumab and convalescent plasma when compared to the group which received convalescent plasma alone 10.However it didn’t reach statistical significance.
We also analyzed the trends of Absolute lymphocyte count and inflammatory markers like CRP, Ferritin and D-dimer between the survivor group and non survivor group. We found that Absolute lymphocyte count was significantly low before Tocilizumab in the non survivor group compared to the survived group indicating that a low ALC on admission could be a marker for poor outcome in patients with severe COVID 19 pneumonia. Absolute lymphocyte count gradually increased and reached the normal level by the end of 2 weeks in survived group whereas it was persistently low in the non survivor group. When comparing the CRP between the survived group and the non survived group we found that CRP was significantly high prior to Tocilizumab administration in both the groups and it drastically decreased in both the groups following Tocilizumab making it an ineffective predictor of clinical outcome post Tocilizumab administration.
When comparing the ferritin value between the survivor group and the non survivor group, we found that ferritin level was gradually decreasing post Tocilizumab administration unlike the CRP levels, which reduced drastically following Tocilizumab. Although the ferritin was decreasing in the survived group compared to the non survivor group, the difference was not statistically significant.
When comparing the D-dimer values between the survivor group and the non survivor group, the D-dimer values were high in both the groups before Tocilizumab. After Tocilizumab administration, the D-dimer values showed a gradual fall in the survivor group similar to the ferritin curve. The significant difference in D dimer fall in the survivor group was observed by the end of 2 weeks to 28 days post Tocilizumab administration. In the study conducted by Hashimoto et al, they observed that Tocilizumab administration rapidly decreased CRP levels followed by a gradual decrease in ferritin and increase in Absolute lymphocyte count 11. The result of this study is consistent with our study results.
Summary
The study was conducted in the Department of General medicine, Kauvery Medical Center Trichy, Tamilnadu from June 2020 to December 2020 on patients who were admitted with COVID 19 pneumonia and received Tocilizumab in the cytokine storm phase of COVID 19 pneumonia. The aims and objectives of our study were to study the mortality rate by day 28 of administration, impact of age, sex, comorbidities and concurrent convalescent plasma transfusion on the outcome post Tocilizumab administration. We also analyzed the trends of ALC, CRP Ferritin and D-dimer between the survived group and the group, which succumbed to death. We found that
- The 28 days mortality rate was 38.3% among the 60 patients who received Tocilizumab.
- The mortality rate was significantly lower in the age group less than 50
- No significant difference in mortality was observed when comparing Male and female gender.
- The presence or absence of comorbidities and the concurrent convalescent plasma transfusion did not significantly influence the mortality.
- Mortality was lower in the group, which received Tocilizumab within 14 days of illness when compared to the group, which received the drug after 14 days.
- Absolute Lymphocyte Count (ALC) was higher before Tocilizumab, 2 days, 1 week and 2 weeks post Tocilizumab administration in the survivor group compared to the non survivor group.
- CRP levels dropped drastically 2 days post Tocilizumab administration with no significant difference in both the survivor and the non survivor groups making it a poor predictor of outcome post Tocilizumab administration.
- Ferritin levels dropped gradually over 2 weeks to 28 days post Tocilizumab administration with no marked difference between the survivor and the non survivor groups.
- The D dimer levels also dropped gradually over 2 weeks to 28 days post Tocilizumab administration but there was a significant difference in D-dimer values between the survivor and the non survivor group from 2nd week onwards.
Conclusion:
Tocilizumab was used in a selected set of patients who had severe COVID-19 pneumonia with acute deterioration. This improved chances of survival in this group of patients when administered at the appropriate time. Younger patients (Age <50) and patients who received Tocilizumab early within 14 days of illness had better survival. Persistent low absolute count was associated with increased mortality. CRP and Ferritin levels reduced quickly in all patients and didn’t predict mortality. D dimer levels reduced in all patients up to 2 weeks of illness but those who did not survive continued to have elevated D dimer levels beyond two weeks of illness. We found significant difference in D-dimer values beyond 2 weeks of Tocilizumab between the survived and the non survived group. The limitations of our study was that it was a retrospective study and we analyzed only a small group of patients.
References
- Nicastri E, et al. Coronavirus disease (COVID-19) in a pauci symptomatic patient: Epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020.Euro Surveill. 2020;252000230.
- Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062.
- Guan W-J, et al. Clinical characteristics of corona virus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Pederson SF. Ho Y-CSARS-Co-2: a storm is raging. J Clin Invest. 2020;130:2202-2205.
- Onder G, et al. Case fatality rate and characteristics of patients dying in relation to COVID 19 in Italy. JAMA. 2020.
- Kopel J, et al. Racial and Gender based differences in COVID 19. Front public health. 2020.
- Miftode E, et al. Diabetes mellitus-A risk factor for unfavorable outcome in COVID 19 patients-The experience of an Infectious disease regional hospital. Healthcare(Basel). 2021;9(7):788.
- Tsai A, et al. Impact of Tocilizumab administration on mortality in severe COVID-19. Sci Rep. 2020;10:19131.
- Diaz R, et al. Does timing matter on Tocilizumab administration? Clinical, analytical and radiological outcomes in COVID 19. Eur J Hosp Pharm. 2021
- Khamis F, et al. The role of convalescent plasma and Tocilizumab in the management of COVID 19 infection. A cohort of 110 patients from a tertiary care hospital in Oman. J Epidemiol Global Health 2021:11(2);216.
- Hashimoto S, et al. Prompt reduction in CRP, IL-6, IFN alpha, IP-10 and MCP-1 and a relatively low Basal ratio of Ferritin/CRP is possibly associated with the efficacy of Tocilizumab monotherapy in severe to critically ill patients with COVID-19. Front Med. 8:734838.

