Cardiovascular effects of sodium – glucose co-transporter- 2 inhibitor, Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl-peptidase 4 inhibitors
N.R. Vijayalakshmi,
Assistant Manager – Clinical Pharmacist, Heart City, Trichy, Kauvery-Heartcity
Introduction
Worldwide, 1-2% of the general adult population have heart failure (HF), which is accompanied by reduced quality of life, high morbidity, mortality and significant financial costs. Existing therapies such as renin angiotensin aldosterone system (RAAS) inhibition, beta-blockade and angiotensin receptor blockers/neprilysin inhibitors (ARNI) reduce hospitalization and mortality risk in patients with HF and reduced ejection fraction. Type 2 diabetes (T2D) is among the many comorbidities associated with cardiovascular disease (CVD) that contributes to end organ damage, and T2D also intensifies the risk for developing HF and HF-related complications, including death [1].
SGLT2 Inhibitors
Sodium-glucose co-transporter-2 (SGLT-2) inhibitors are antihyperglycemic agents acting on the SGLT-2 proteins expressed in the proximal convoluted tubules. These drugs exert their effect by preventing the reabsorption of filtered glucose from the tubular lumen. To date, there are four SGLT-2 inhibitors: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin that are approved by Food Drug Administration (FDA) for their use in adults.
FDA-approved indications for SGLT-2 Inhibitors
Improvement of glycemic control in type 2 diabetes mellitus (adjunct to diet and exercise)
Reduction of major adverse cardiovascular events (nonfatal myocardial infarction and nonfatal stroke, cardiovascular death) in patients with type 2 DM and established cardiovascular disease.
To decrease the risk of cardiovascular hospitalization and death for heart failure in patients with HFrEF (heart failure with reduced ejection fraction-NYHA class II-IV)
Dapagliflozin is now FDA-approved for the treatment of heart failure across the full spectrum of left-ventricular ejection fraction (LVEF), including HFrEF, HFpEF, and HFmrEF (Heart failure with mildly reduced ejection fraction- LVEF of 40-49%)
Off-Label use of SGLT2 Inhibitors
Management of obesity in combination with glucagon-like peptide -1 receptor agonists
Nonalcoholic fatty liver disease (NAFLD): AACE recommends SGLT2 inhibitors as adjunctive therapy in patients with type 2 DM and NAFLD [2].
Mechanism of action
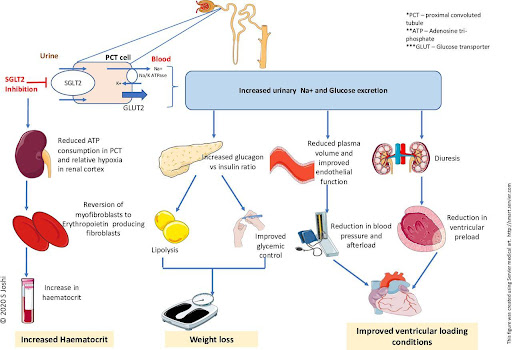
Administration of SGLT2 inhibitor
SGLT2 inhibitors are administered in oral formulations.
Canagliflozin is available in 100 mg and 300 mg tablets. Canagliflozin is administered before the first meal of the day.
Dapagliflozin is available in 5 mg and 10 mg
Empagliflozin is available in 10 mg and 25 mg tablets. Empagliflozin is taken once daily in the morning
Ertugliflozin is available in 5 mg and 15 mg tablets. Ertugliflozin is administered once daily in the morning.
Drug-Drug Interactions and Drug/Laboratory Test Interference
The risk of hypoglycemia increases when SGLT2 inhibitors are combined with an insulin secretagogue (eg, sulfonylurea) or insulin. Therefore, clinicians should reduce the insulin/insulin secretagogue dose to decrease the risk of hypoglycemia.
SGLT2 inhibitors, including empagliflozin, decrease sodium-glucose and lithium-glucose reabsorption in the proximal connecting tubules, thereby increasing the renal excretion of sodium, glucose, and lithium. Concurrent use of an SGLT2 inhibitor with lithium can reduce serum lithium concentrations. .
UGT enzyme inducers, such as rifampin, phenytoin, ritonavir, and phenobarbital, reduce canagliflozin exposure (AUC) which may reduce the effectiveness of canagliflozin. Consider increasing the dose of canagliflozin when used with UGT enzyme inducers [2]. .
Glucagon-like Peptide-1 Receptor Agonists
Glucagon-like peptide-1 (GLP-1) agonists (also known as GLP-1 receptor agonists, incretin mimetics, or GLP-1 analogs) represent a class of medications used to treat type 2 diabetes mellitus and, in some cases, obesity. Examples of drugs in this class include exenatide, lixisenatide, liraglutide, albiglutide, dulaglutide, and semaglutide. Glucagon-like peptide-1 is derived from human gut hormones. While originally found to have insulinotropic effects (i.e. increased insulin secretion due to rising blood glucose levels) , a property which has led to the development of several potent GLP-1 based drugs which improve HbA1c up to 2% , evidence has arisen to show that the hormone has non-glycaemic effects as well . Since the discovery of GLP-1 receptors (GLP-1Rs) on cardiomyocytes, possible CV benefits of GLP-1 and GLP-1 based therapies have spiked the attention of many clinicians and researchers. Small-sized clinical trials demonstrated possible beneficial effects of GLP-1 and GLP-1 based therapies on cholesterol levels, blood pressure (BP), the microcirculation and low-grade inflammation. More importantly, large randomised CV outcome trials (CVOTs) demonstrated that GLP-1 receptor agonists (GLP-1RAs) reduce CVD in T2DM patients.
Mechanism of action:
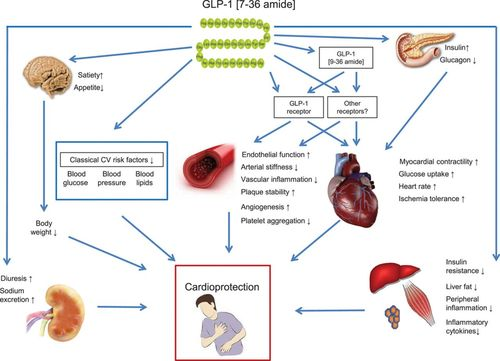
Administration of Glucagon-like Peptide-1 Receptor Agonists:
Many formulations of GLP-1 agonists, all of which historically were injectable and administered subcutaneously due to poor oral bioavailability, can be prescribed in the United States. Lixisenatide and liraglutide dosing are once-daily, albiglutide, dulaglutide, semaglutide dosing is once weekly, and exenatide can be dosed either as a twice-daily or a once-weekly injection. Recently, the FDA approved an oral formulation of semaglutide.
Dipeptidyl-peptidase 4 inhibitors:
DPP-4 inhibitors, known as gliptins, are a class of oral diabetic medications approved by the Food and Drug Administration (FDA) to treat type 2 diabetes mellitus in adults. DPP-4 inhibitors that have FDA approval include sitagliptin, saxagliptin, linagliptin, and alogliptin. Vildagliptin has approval from the European Medicines Agency (EMA), but not by the FDA [5]. They appear to exert beneficial effects on other established cardiovascular risk factors, including dyslipidemia and hypertension. Moreover, DPP-4 inhibitors exert antiinflammatory and antioxidant actions, improve endothelial function and reduce urinary albumin excretion.
Role of DPP4 in cardiovascular disease:
There is good evidence that DPP4 inhibition mediates protective effect on myocardial infarction, hypertension, and atherosclerosis. Pharmacologic inhibition with sitagliptin also enhances expression of cardioprotective proteins and improved functional recovery after I/R injury in the murine heart. Several studies in animal models support a favorable effect of DPP4 inhibition in improving endothelial function and blood pressure. Improved left ventricular function assessed by ejection fraction, mitral annular systolic velocity, and peak systolic velocity was also observed in humans with DPP4 inhibition (sitagliptin, single dose or 4 weeks of treatment). DPP4i are safe from cardiovascular perspective although no significant improvement was observed in cardiovascular endpoints on T2DM patients with antidiabetic medications [5].
Mechanism of action
DPP-4 is a ubiquitous enzyme that acts on incretin hormones, mainly GLP-1 (glucagon-like peptide-1) and GIP (gastric inhibitory peptide), which maintain glucose homeostasis by increasing insulin secretion and decreasing glucagon secretion. GLP-1 is a hormone secreted by enteroendocrine L cells of the small intestine, which lowers blood glucose by stimulating insulin secretion, reducing glucagon concentrations, and delaying gastric emptying. It has a half-life of fewer than 2 minutes. GIP is a hormone secreted in the stomach and proximal small intestine by neuroendocrine K-cells. Its half-life is approximately 7 minutes in healthy individuals and 5 minutes in individuals with type 2 diabetes. These incretins are released within minutes of food intake, and DPP-4 degrades these hormones immediately due to their short half-life. By inhibiting the DPP-4 enzyme, DPP-4 inhibitors increase the levels of GLP-1 and GIP, which in turn increase beta-cell insulin secretion in the pancreas, thereby reducing postprandial and fasting hyperglycemia [6].
Conclusion
Diabetes mellitus individuals are more likely to experience cardiovascular disease, which continues to be the leading cause of mortality and disability in these patients. Renin-angiotensin-aldosterone, ACE inhibitors/ARBs/MRAs, and -blockers in the sympathetic nervous system have traditionally been the mainstays of heart failure therapies. In patients with HFrEF, including those who have diabetes, these medications have decreased mortality and hospitalization for heart failure. Recently, however, newer therapies such as SGLT2 inhibitor, glucagon-like peptide-1 receptor agonists, and dipeptidyl-peptidase 4 inhibitor have been widely used in T2DM, which have been proven to be safe for cardiovascular disease. These medications have been shown to reduce the risk of cardiovascular events, particularly heart failure, and cardiovascular death. Its importance to note that individual patient factors, such as comorbidities and medication tolerability, should also be taken into consideration when selecting the appropriate treatment for a patient with type-2 diabetes and cardiovascular concerns.
References
- Lytvyn Y, et al. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017;136(17):1643-1658.
- Padda IS, et al. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. [Updated 2023 May 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- https://www.elsevier.com/__data/assets/pdf_file/0011/1146962/Sodium-Glucose-Cotransporter-2-SGLT2-Inhibitors_MAR-16-2021.
- Heuvelman VD, et al. Cardiovascular effects of glucagon-like peptide 1 receptor agonists: from mechanistic studies in humans to clinical outcomes. Cardiovasc Res. 2020 Apr 1; 116(5):916-930.
- Kasina SVSK, et al. Dipeptidyl Peptidase IV (DPP IV) Inhibitors. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Weijia Xie, et al. Impact of dipeptidyl-peptidase 4 inhibitors on cardiovascular diseases. 2017

