Management of germ cell tumors: A review
Sneha1*, G. Surendra kumar2
1Staff Nurse, Kauvery Hospital, Tennur, Tamilnadu
2Surgical Oncologist, Kauvery Hospital, Tennur, Tamilnadu
Background
Malignant ovarian germ cell tumors (MOGCTs) are rare. Unlike epithelial ovarian cancer, MOGCTs typically occur in girls and young women. Fertility-sparing surgery and platinum-based chemotherapy remain the standard of care, providing high chance of cure at all stages. Given the lack of high-quality studies in this field, current practice guidelines recommend chemotherapy regimens adopted in testicular germ cell tumors. However, platinum-resistant/refractory MOGCTs retain a worse prognosis in comparison with their male counterpart. Herein, we focus on current systemic anti-cancer treatment options in MOGCTs and promising approaches.
Epidemiology
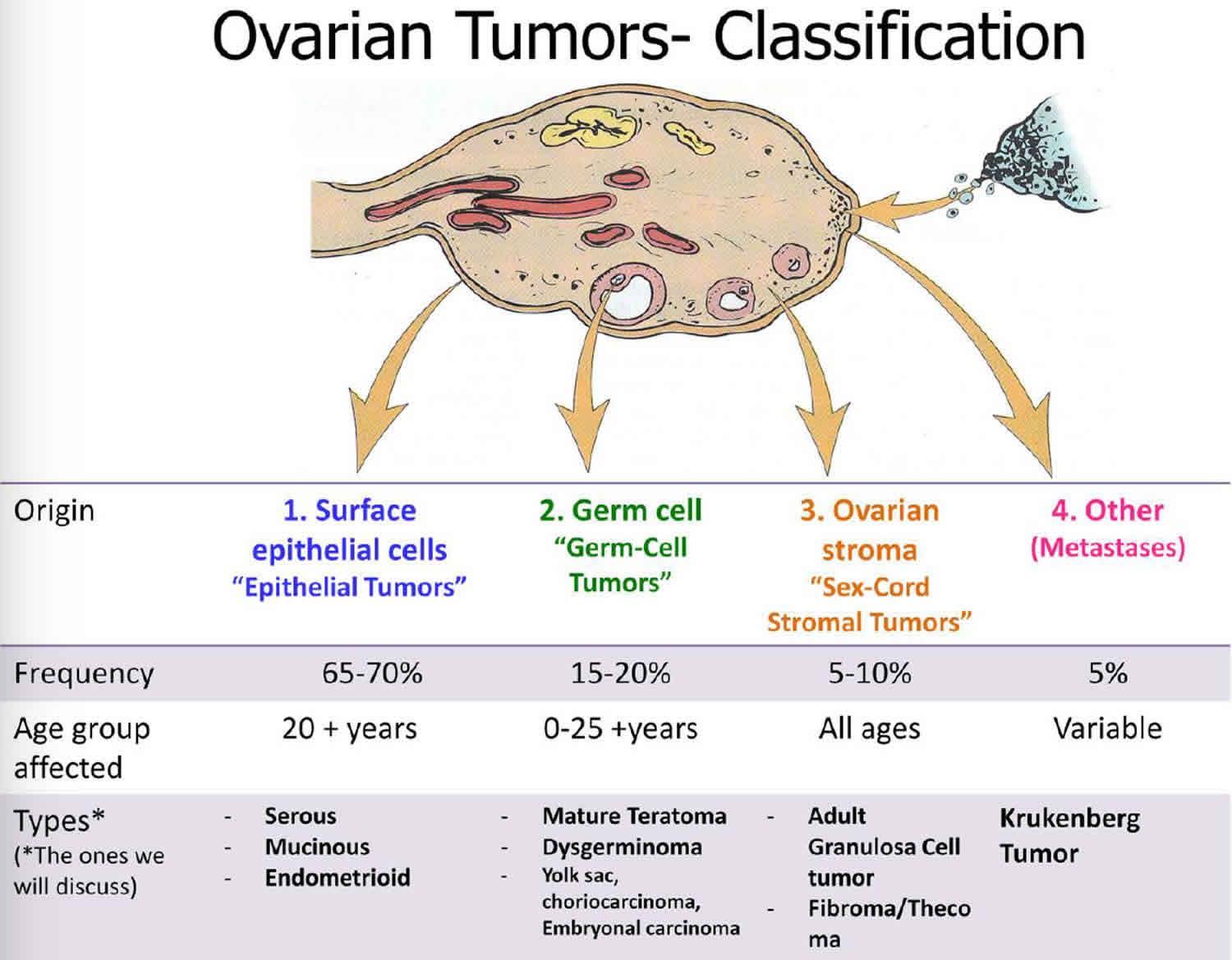
Malignant ovarian germ cell tumors (MOGCTs) account for approximately 1-2% of all ovarian malignancies.1 In women, MOGCTs account for 20-25% of all ovarian neoplasms, but only 3-5% of these are malignant.7, 8. The peak incidence is in young women or adolescent girls and germ cell tumors (GCTs) account for 58% of all ovarian tumors in women younger than the age 20 yr, one third of these tumors are malignant.
Clinical presentation
The most common symptom is abdominal pain occurring in 55-80% of the patients. Other signs comprise the presence of abdominal or pelvic mass with abdominal enlargement. Abdominal pain associated with a palpable pelvic-abdominal mass is observed in about 85% of patients. Fever is present in 10-25% of the cases. Ascites or peritonitis secondary to torsion, infection, or rupture of the ovarian tumor, are other possible clinical features.
The stage distribution at diagnosis is particularly different from that of epithelial tumors. Approximately 60-70% of cases are FIGO stage I or II, 20-30% are stage III, and stage IV is relatively uncommon. Bilateral ovarian involvement is uncommon, except in the case of dysgerminomas. Bilateral involvement occurs in about 10-15% of dysgerminoma patients.
Diagnosis /pathology and tumor marker
Tumor marker
Tumor markers play an important role for the diagnosis and management of MOGCTs, used to check for complete remission or recurrence. Elevated pre-operative levels of a-fetoprotein (AFP) and b-human chorionic gonadotrophin (b-HCG) are virtually diagnostic of a MOGCT and must be measured in all young women that present with a pelvic mass. Mixed germ cell tumors may produce either both or none, depending on the type and quantity of elements present

Pathology
The World Health Organization (WHO) classification of GCT is presented GCTs with extraembryonic differentiation are all malignant. Teratomas are the most common GCTs; most are composed of mature tissues and are benign (dermoid cysts). In immature teratomas, embryonic tissues indicate the malignant potential and grading is prognostically relevant[2]. Grade 1 tumours show rare foci of immature neuroepithelial tissue that occupy < 1 low power field in any slide (low grade); grade 2 tumours show similar elements, occupying 1-3 low power fields in any slide (high grade); grade 3 tumours exhibit large amounts of immature neuroepithelial tissue occupying >3 low power fields in any slide (high A two-tiered, low and high grades) system is now more commonly used.
Diagnosis can be made on conventional histological material; given the multiplicity of morphological features, immunohistochemical markers and chromosome 12p fluorescent in situ hybridisation (FISH) can be used to confirm the diagnosis in difficult cases. SALL4 and OCT4 are widely used; more recently, it has been recognised that SOX2 is expressed in embryonal carcinoma and primitive neuroectodermal tumours of teratomatous origin.
Staging
- The American Joint Committee on Cancer (AJCC) tumour-node-metastasis (TNM) classification and the International Federation of Gynaecology and Obstetrics (FIGO) staging system for germ cell tumours are listed below .Malignant germ cell tumours of the ovary follow the same staging system as epithelial ovarian and primary peritoneal cancers. The staging system for non-epithelial ovarian cancers is generally adopted from the one for epithelial ovarian cancer originally defined by the International Federation of Gynaecology and Obstetrics (FIGO)
- Outcomes may depend on the age at diagnosis. Premenarchal girls and women > 45 years who develop GCTs may have different tumour biology and a worse prognosis than post adolescent females in the reproductive years.
- Adverse factors include age >45 years, stage>I, incomplete surgical resection and yolk sac tumour (YST) histology.
- A surgical approach can be carried out through open route or, in selected cases, by minimally invasive approaches-laparoscopy and robotics-to avoid tumour rupture during surgery. A careful examination of the abdominal cavity is required. The staging procedure includes infracolic omentectomy, biopsy of the diaphragmatic peritoneum, paracolic gutters, pelvic peritoneum and peritoneal washings in macroscopic stage I disease.
Germ cell tumours
Unilateral salpingo-oophorectomy with preservation of the contralateral ovary and the uterus are now considered as the standard surgical treatment for young patients with GCTs
This conservative management should be considered even in the case of advanced disease because of the sensitivity of the tumour to ChemoTherapy ( ChT). Systematic ovarian biopsy is not necessary when the contralateral ovary is macroscopically normal. In the case of macroscopic bilateral ovarian diseases (particularly dysgerminoma or immature teratoma), preservation of at least a healthy part of one ovary (unilateral salpingo-oophorectomy and contralateral cystectomy) and the uterus should be encouraged. In postmenopausal women and patients with advanced-stage disease or with bilateral ovarian involvement, abdominal hysterectomy and bilateral salpingo-oophorectomy could be carried out with careful surgical staging.
Management of early stages
Germ cell tumours
Most GCTs (60%-70%) are diagnosed early. Stage I patients have an excellent prognosis with long-term disease-free status of about 90%. Given the young age of patients, all efforts should be made to preserve fertility. Fertility-sparing surgery appears to be safe with excellent survival after long-term follow-up, yielding outcomes equivalent to patients undergoing hysterectomy with bilateral salpingo-oophorectomy.
Management of advanced disease
Fertility-sparing surgery should also be considered in advanced stages disease as cure rates remain high. The aim of surgery is to remove as many gross tumours as possible; however, the procedure should be moderated to avoid delays in postoperative ChT.

Toxicity of BEP and long term complications
- Pulmonary toxicity, Decreased DLCO
- AML
- Neuropathy
- Raynaud’s disease
- Tinnitus
- High tone hearing loss
- Gonadal dysfunction
- Cardiovascular disease/Hypertension
- Nephrotoxicity
- Osteoporosis after radical surgery for young patients.
Advanced-stage and recurrent GCTs: Management
- Fertility-sparing surgery should be considered also in advanced stages [IV, B]. Surgery aims to remove as much gross tumour as possible; however, the procedure should be moderated to avoid delays in postoperative ChT and long-term morbidity.
- In postmenopausal women with advanced-stage disease or with bilateral ovarian involvement, abdominal hysterectomy and bilateral salpingo-oophorectomy could be carried out with careful surgical staging [III, A].
- Platinum-based regimens are the treatment of choice with the BEP regimen being the most widely used. Generally, three cycles of a 5-day BEP regimen in completely resected disease and four cycles (bleomycin should be omitted to reduce the risk of lung toxicity after the third cycle) for patients with macroscopic residual disease.

Follow-up, long-term implications and survivorship
- For patients undergoing ChT, serum tumour markers (Hcg, a-FP, LDH, CA 125, and inhibin B) can accurately correlate with tumour response during ChT A CT scan of the abdomen, pelvis, and chest (in case of suspected lung metastases). Pelvic ultrasound is the most common and useful imaging technique to evaluate the response to ChT in patients with measurable disease.
- Follow-up visits for GCT patients must include history, physical and pelvic examinations and exploration of tumour markers every 3 months for the first 2 years, every 6 months during the third year and then yearly until progression [V, A]. Tumour marker exploration can be avoided from the third year.
Hormone replacement therapy and contraception
HRT is an effective treatment mainly used for the treatment of vasomotor symptoms, low mood, sexual dysfunction and urogenital symptoms following menopause. Moreover it may dramatically improve physical and psychological symptoms and in the end, the quality of life in cancer patients.

