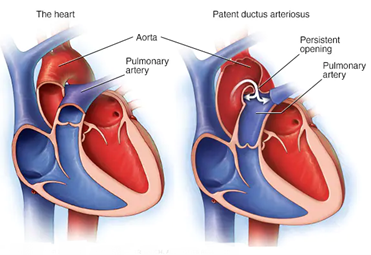
Patent Ductus Arteriosus (PDA)
Hema
Echo Technician, Kauvery Hospital, Heart city, Trichy
Abstract
Patent ductus arteriosus (PDA) is a heart condition commonly seen in pediatrics. It happens when a blood vessel called the ductus arteriosus does not close, as it should after birth. PDA occurs when the opening between the aorta (the artery that carries oxygen-rich blood to the body) and the pulmonary artery (the artery that carries oxygen-poor blood to the lungs) does not close as it should. The persistent opening causes too much blood to flow to the baby’s lungs and heart. Untreated, the blood pressure in the baby’s lungs might increase. The baby’s heart might grow larger and get weak.
In this article is going to discuss the causes, types, symptoms, diagnosis in echo, treatment and prevention of PDA.
Introduction
Patent ductus arteriosus (PDA) is a persistent opening between the two major blood vessels leading from the heart. The heart problem is present from birth. That means it is a congenital heart defect. During the first six weeks of pregnancy, a baby’s heart starts to form and beat. The major blood vessels to and from the heart grow. It’s during this time that certain heart defects may begin to develop. Before birth, a temporary opening called the ductus arteriosus is between the two main blood vessels leaving a baby’s heart. Those vessels are the aorta and the pulmonary artery. The opening is necessary for a baby’s blood flow before birth. It moves blood away from a baby’s lungs while they develop. The baby gets oxygen from the mother’s blood. After birth, the ductus arteriosus is no longer needed. It usually closes within 2 to 3 days. But in some infants, the opening doesn’t close. When it stays open, it’s called a patent ductus arteriosus.
A small patent ductus arteriosus often doesn’t cause problems and might never need treatment. However, a large, untreated patent ductus arteriosus can let oxygen-poor blood move the wrong way. This can weaken the heart muscle, causing heart failure and other complications.
Causes of PDA
Causes of this condition is not sure. Patent ductus arteriosus causes may include genetic disorders or a family history of the condition. This is much more common in premature infants (babies born more than three weeks before the projected due date). Studies suggest PDA affects about 65% of infants born before the 28th week of pregnancy. It is rare in full-term babies and is twice as common in girls than in boys.
Sometimes PDA occurs with other heart defects. The risk of congenital heart defects like PDA may also increase due to:
- Certain genetic conditions
- Family history of congenital heart conditions
- Fetal distress in the womb
- Infections in the mother or fetus during pregnancy, such as rubella
- Other pregnancy-related risk factors, such as smoking or taking certain medications
- German measles during pregnancy: Babies born to mothers who had rubella (German measles) during pregnancy may have a higher risk of a PDA.
- Neonatal respiratory distress syndrome: Babies whose lungs didn’t get enough lubricating substance (surfactant) before birth may develop neonatal respiratory distress syndrome, a breathing problem. These babies may also develop a PDA.
Types of PDA
During fetal life, the ductus is a normal structure that permits blood leaving the right ventricle to bypass the pulmonary circulation and enter the descending aorta. Less than 10% of this blood enters the pulmonary circulation. After birth, the ductus closes within 24-48 hours. The ductus is a remnant of the distal sixth aortic arch and connects the proximal descending aorta to the main pulmonary artery. The ductus can be found just posterior to the arch of the aorta where it enters the anterior pulmonary artery. The ductus has a conical shape which is large at the aortic end and narrow at the pulmonary end. However, the shape, size, and length of the ductus are very variable.
For surgeons, an anatomical marker of the patent ductus is the recurrent laryngeal nerve which loops posteriorly around the ductus and ascends behind the aorta en route to the larynx. The recurrent laryngeal nerve is often injured during surgical ligature of the ductus.
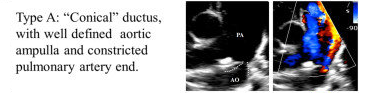
The Patent ductus is classified based on its angiographic features and includes the following:
Type A: Conical
Type B: Window

Type C: Tubular

Type D: Saccular

Type E: Elongated

Type F: Fetal

Symptoms
Symptoms of PDA depend on the size of the opening between a baby’s aorta and pulmonary artery. symptoms vary according to patent ductus arteriosus types. Small PDAs may not cause any symptoms other than a heart murmur.
A large PDA found during infancy or childhood might cause:
- Rapid breathing
- Shortness of breath (dyspnea).
- Sweating during feedings.
- Fatigue or tiredness.
- Feeding and eating problems.
- Poor weight gain or growth.
- Fast pulse or heart rate.
- Fast or hard breathing
- Frequent respiratory infections
- Heart murmur (a “whooshing” sound made by abnormal blood flow through the heart)
- Poor weight gain
- Trouble feeding or tiredness while feeding
Some people don’t notice symptoms until adulthood. A large PDA can cause symptoms of heart failure soon after birth.
ECG Changes
The ECG may demonstrate sinus tachycardia or atrial fibrillation, left ventricular hypertrophy, and left atrial enlargement in patients with moderate or large ductus shunts. In patients with smaller ductal shunts, the ECG is often completely normal.

- With a small shunt the ECG is normal
- Left ventricular hypertrophy of the volume overload type, with deep O Waves and increased R wave voltage in the left precordial leads, is noted with increasing shunt size with left ventricular volume overload
- Right ventricular hypertrophy is seen with pulmonary hypertension
Diagnosis by Echo
The echocardiography is the gold standard bedside investigation to diagnose PDA. In addition, to make a confirmative diagnosis of PDA and exclude/diagnose any associated congenital heart defect (CHD). It can help in estimating the magnitude of shunt volume and assessing its hemodynamic significance—it can be used to assess the hemodynamic impact from pulmonary over circulation and systemic hypo perfusion due to shunt volume This could be systematically achieved by studying (a) ductal characteristics, (b) parameters of pulmonary over circulation, and (c) signs of systemic hypo perfusion.
The echocardiography can be used to assess the size of PDA by measuring trans-ductal diameter, interrogate shunt direction, and velocity of blood flow across the ductus arteriosus can be measured by using Doppler technique.

Complications
- Heart failure
- Endocarditis
- Pulmonary edema (fluid in the lungs)
- Pulmonary hypertension
Treatment
Healthcare provider will consider baby’s age, size and health when determining a treatment plan. They might recommend observation to see if the PDA will close on its own. If a small PDA does not cause severe symptoms, it may not need treatment. Sometimes the connection may close on its own a few months after birth. A baby may need medicine such as indomethacin (an anti-inflammatory) during these months to help close the connection, or water medicine (diuretics) to reduce the risk of fluid buildup. Larger connections usually need treatment with catheterization or surgery.
PDA (Device Closure)
Baby of Hashini was diagnosed with a PDA issue when she was just 12 days old. Her parents consulted pediatric cardiologist, and was on regular check-ups & follow-ups.
When she was 5 years old, her health condition was discussed with consultant, pediatric cardiologist. After carefully examining the entire situation, pediatrician advised PDA device closure surgery for the child.
The PDA causes lots of complication to the patient, therefore it necessitates immediate medical attention. However, the patient’s family showed complete faith in the doctor’s prognosis and agreed for the surgery.
After the successful completion of PDA device closure surgery, the patient and her family were happy to seeing the minimally invasive procedure. She was discharged within a couple of days and is now doing well in her life.

Echocardiography: Suprasternal view with PDA, from preoperative.
PDA: Patent ductus arteriosus; occlude: the device closed the defect without residual shunt.
Conclusion
For infants with just an isolated PDA, the prognosis is good. In premature infants, the prognosis is dependent on other comorbidities. After closure the PDA, most children have a normal life expectancy. Spontaneous closure of the PDA is rare, with the use of indomethacin; close to 80-90% of infants will have successful closure of the PDA. In adults, a surgical closure is always required provided the patient has not developed fixed pulmonary hypertension.

