Retroperitoneal sarcoma: A report of two cases
Patne SKS1, Menon A2, Manje Gowda D3, Nagaraja R4*
[1, 2, 3] Department of Gastroenterology, Kauvery Hospital Marathahalli, Bengaluru
4Consultant and Head, Surgical Gastroenterology/HPB/Liver transplantation, Kauvery Hospital Marathahalli, Bengaluru
Abstract
Retroperitoneal sarcomas are rare tumors, with the potential for late presentation due to their location, lack of significant symptoms at an early stage and availability of space for expansion. For the same reason, along with their proximity to vital structures, the majority of such tumors are deemed inoperable. Surgery offers the only hope of cure in such patients, at times with the addition of adjuvant therapy. We present here two cases of large retroperitoneal sarcomas where complete resection was possible, with potential for cure.
Keywords: Liposarcoma; Leio-myo sarcoma; Retro-peritoneum; RPS retro-peritoneal sarcoma.
Introduction
The retroperitoneum can host a wide spectrum of soft tissue lesions. Approximately 70–80% of primary retroperitoneal soft-tissue tumors are malignant; however, these account for just 0.1–0.2% of all malignancies.[1] It is better to exclude malignancy considering the age, size, imaging appearance, vascularity and other features to attain better outcomes in such patients.
Most of the malignant tumors occurring in the retroperitoneum are RPS. Although more than 70 histological types of sarcomas have been identified, liposarcoma, leiomyosarcoma and undifferentiated pleomorphic sarcoma, formerly known as malignant fibrous histiocytoma, account for the vast majority of RPS. [2]
These lesions remain silent till late stages due to their location in the dorsal aspect of abdominal cavity, absence of specific symptoms and availability of large potential space for expansion. Due to their late identification, large size and closeness to vital structures, most of them are considered inoperable. Surgery offers the only hope of cure in such patients with need of adjuvant therapy in some.
We report here, two cases of retroperitoneal sarcoma, managed with a curative intent with surgery.
Case Presentation
Case 1
A 54-year-old female presented with a three-week history of an abdominal lump involving the entire right half of the abdomen, loss of appetite and significant weight loss. She was hypertensive, but well-controlled on low-dose anti-hypertensives for 6 months. Her vitals were normal.
On abdominal examination, a large, solid, non-tender mass was palpable involving the entire right hemi-abdomen, which was bimanually palpable and ballotable.
Laboratory evaluation was normal and non-contributory.
A contrast CT scan of abdomen (Fig 1), revealed a large, hyper vascular, heterogeneously enhancing soft tissue mass lesion in the retro-peritoneum on right side. Encasing the lower pole of the right kidney and inseparable from the renal parenchyma, with significant mass effect over the adjacent structures and displacing adjacent bowel loops to the left with loss of intervening fat planes and moderate right hydronephrosis.
Imaging features were suggestive of retro-peritoneal sarcoma versus renal cell carcinoma. Percutaneous cytology or biopsy was not considered in view of risk of bleeding in such a vascular tumor. The endocrine evaluation was not considered in view of imaging findings and not being symptomatic for same. After metastatic workup, she was taken up for resection.
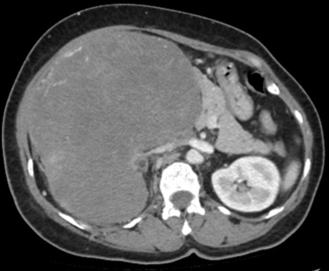
Fig (1): CT Abdomen showing the large tumour engulfing the right kidney and abutting the vital surrounding structures
At laparotomy, a large well encapsulated, vascular mass occupying the whole of right hemi-abdomen was found, engulfing the right kidney and ureter, pushing bowel anteriorly and to left. Anteriorly it was densely adherent to right colon, duodenal loop and pancreas, posteriorly to the ilio- psoas muscle and medially to the vena cava.
Surgery was difficult due to size, vascularity and dense adhesions to vital structures. Right kidney was not salvageable. Tumor was completely resected, without spillage/fractionation Estimated, intra-operative blood loss was 1000ml and she received 2 units of PRBC. She had an uneventful postoperative recovery and was discharged home on postoperative day 5.
On gross examination the mass weighed 3.6 kg, measuring 25 × 20 ×16 cm in dimension and looked homogenous, fleshy with both solid and cystic components with high vascularity abutting and engulfing right kidney (Fig 2).
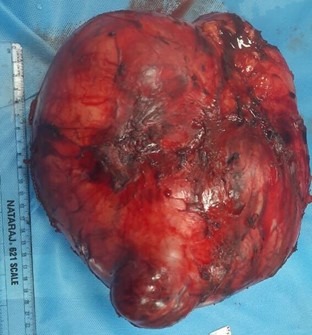
Fig (2): Post resection specimen picture
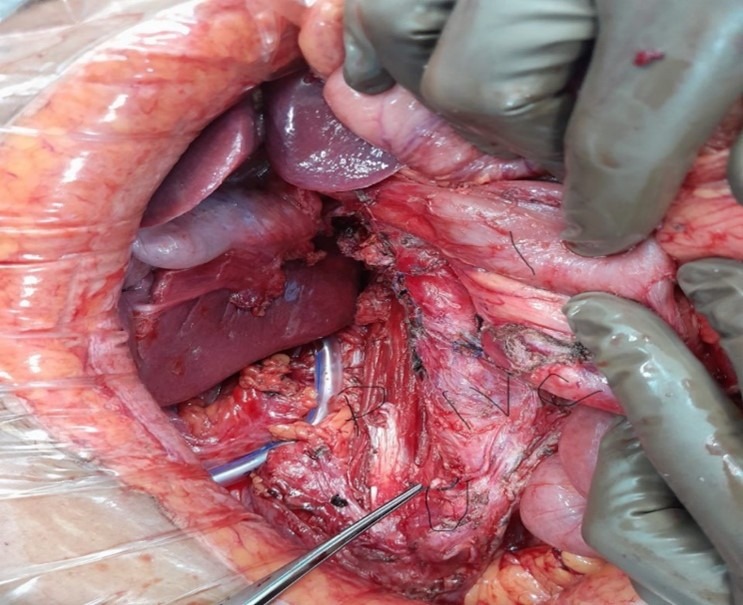
Fig (3): Post resection tumor bed pic. P- Psoas, U- tied end of ureter, IVC, I- intestines
On microscopic examination (Fig 4 and 5), the tumour was composed of varying sized spindle shaped cells ranging from slender ones to plump and epithelioid, arranged haphazardly and as short fascicles within a collagenous to edematous stroma containing moderate interstitial and perivascular inflammatory infiltrate.
Towards the periphery of the tumour were seen clusters of adipocytes of varying sizes with focal nuclear atypia, suggestive of a well differentiated liposarcoma component. Definite lipoblasts were not seen. Ureter and renal vessels were free from tumour.
The tumour showed focal positivity for SMA and Desmin. Many pleomorphic tumor cell nuclei were positive for MDM2.

Fig (4): Atypical spindle cells with well-differentiated liposarcoma component
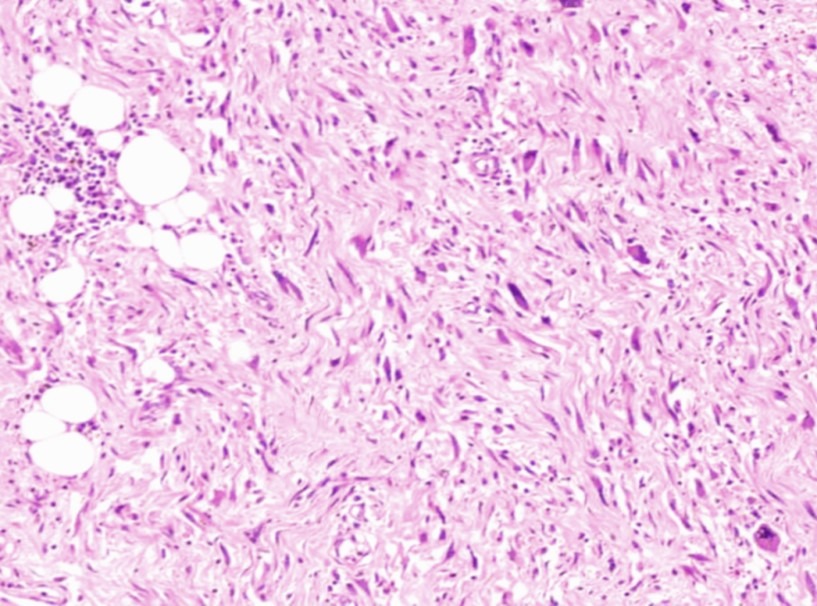
Fig (5): Atypical spindle cells with background inflammatory cell infiltrate imparting an inflammatory myofibroblastic tumour-like appearance
Case 2
A 56-year-old female was evaluated elsewhere for abdominal pain and found to have left adrenal lesion, for which she was referred to us. Endocrine evaluation, both clinically and laboratory, were negative.
On the CECT abdomen, she was found to have left suprarenal heterogeneously enhancing lesion 7×5×4 cm, closely abutting celiac axis and superior mesenteric artery.
She was taken up for laparoscopic left adrenalectomy, but had to be converted to open surgery in view of dense adhesions. Lesion was found to be hard with significant peri-tumoral desmoplastic/fibrotic reaction. Dissection was difficult since it was densely adherent to abdominal aorta at the exit point of celiac, superior mesenteric and left renal arteries; the former two could be saved and left kidney was taken with the tumor. Intra-operative blood loss was 300cc and she did not require transfusion. She made an uneventful recovery.
On microscopic examination (Fig 6), it showed cellular neoplasm with spindle cells arranged in fascicles, cells with hyperchromatic nuclei, moderate degree of pleomorphism. Areas of necrosis seen and atypical mitotic figures up to 5/10 hpf. Lesion was surrounded by normal looking smooth muscle cells. There was no perineural invasion/ invasion of kidney/ adrenals. It was reported as well as differentiated Leiomyosarcoma.
She received adjuvant radiotherapy one month after surgery and 2 years later, she is doing well with no evidence of disease.
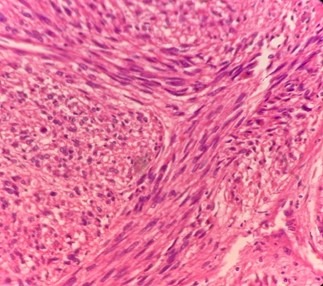
Fig (6): Leiomyosarcoma cellular neoplasm with spindle cells arranged in fascicles, cells with hyperchromatic nuclei, moderate degree of pleomorphism.
Discussion
Retro-peritoneal tumours are extremely rare tumors occurring in the retroperitoneum bounded anteriorly by the posterior parietal peritoneum and posteriorly by the transversalis fascia.[3]
Approximately 70–80% of primary retroperitoneal soft-tissue tumors are malignant; however, these account for just 0.1–0.2% of all malignancies.[1] Most of the malignant tumors occurring in the retroperitoneum are RPS. Although more than 70 histological types of sarcomas have been identified, liposarcoma, leiomyosarcoma and undifferentiated pleomorphic sarcoma formerly known as malignant fibrous histiocytoma account for the vast majority of RPS.[2]
It is most commonly seen in males in their 4 and 5th decade of life. Around 40% of all the retroperitoneal tumours is liposarcoma.[4] As per WHO classification, it has 5 subtypes namely, well differentiated, myxoid, round cell, pleomorphic and dedifferentiated types. About 10% of these tumours dedifferentiate and make the diagnosis difficult.[5]
Even though it appears that dedifferentiated liposarcoma would develop from well differentiated liposarcoma, many cases are found de novo as well.[6] Dedifferentiation is a process of progression from well well-differentiated form to a higher-grade less differentiated form. In most circumstances, dedifferentiation worsens the prognosis. However, in cases of dedifferentiated liposarcoma, neither the local extent nor the grade has any significant influence on the behavior or prognosis of this tumour.[6-8] This type has a vague prognosis compared to other types of sarcoma, and making the histological diagnosis can be difficult. Dedifferentiated liposarcoma commonly develops in the retroperitoneum, limbs, testis and spermatic cord, yet there are only a few such case reports.
Thway et al [9] described the utility of immunohistochemistry for MDM2, CDK4 and p16 in the routine diagnosis of dedifferentiated liposarcoma. However, there have been conflicting studies showing lesser usefulness of p16 as compared to MDM2and CDK4.[10]
For treatment, complete removal of retroperitoneal sarcoma is the most effective point and radical complete surgical removal has a remarkable effect on the survival rate. Complete surgical removal is often difficult and involves removal of adjacent structures in upto 75% of patients, most common being kidney, colon and duodenum. The rate of complete removal is 50% on average.[11] Radiation therapy or additional chemotherapy can be considered for the poorly differentiated sarcoma that is more than more than 10 cm in size or for the cases of incomplete removal.
Leiomyosarcoma is the second most common type of retroperitoneal sarcomas, with an incidence of approximately 20%, while liposarcoma, the most common type of retroperitoneal sarcoma has an incidence of 64%.[12 13]
Symptoms of sarcoma of the abdomen and retroperitoneum vary greatly depending on tumour site. There might be diffuse symptoms or no symptoms at all. Depending on tumour location, there might be haemorrhage, pressure symptoms, pain or ascites [14]. According to Clark et al., the most common finding at diagnosis is a painless, gradually enlarging mass [15]. Some patients primarily present with weight loss and abdominal pain, other with intestinal obstruction and dysphagia. While unspecific, anaemia is also a possible symptom [16].
The National Comprehensive Cancer Network (NCCN) guidelines for intraabdominal and retroperitoneal soft tissue sarcoma, recommends CT of the chest, abdomen and pelvis with intravenous contrast for diagnosis, occasionally supplemented by MRI of lesions in the pelvis or abdomen. PET/CT can be considered in order to detect distant metastases, or to help determine the site of biopsy [17].
The risk of needle tract seeding during this procedure is not zero, but very low, and the benefits of proper preoperative diagnostics are considered to greatly outweigh the risks. 18 We did not consider preoperative biopsy in our patients as it was very vascular in one patient and inaccessible in other.
Recommendations from the NCCN argue that image guided core needle biopsy should be performed if preoperative treatment is planned, or if non-sarcoma malignancies are suspected. If the tumour is a well differentiated liposarcoma, biopsy is unnecessary. The rationale for biopsy is to determine whether the tumour is malignant or benign, provide a specific diagnosis if possible, and determine tumour grade where appropriate.
Leiomyosarcoma can be differentiated from other soft tissue sarcomas by the presence of smooth muscle cell actin and desmin on immunohistochemistry.
The aim of a complete resection is to achieve negative margins in the histological sample. The width of these margins are not ultimately defined in the literature, but some suggest a margin of 1 cm, or a layer of intact fascia.[19] Excessive lymph node resection does not seem to be necessary, as leiomyosarcoma rarely are metastatic to local lymph nodes [20]. If the tumour involves or originate from a blood vessel, the proximal and distal end of the resection should have negative margins. Furthermore, it’s recommended to resect tumour thrombosis if present, but the evidence grade of this is unknown. In addition to the diagnosis being obvious at exploration (containing fat), we found liposarcoma to be well defined and encapsulated, while leiomyosarcoma to be rather greyish, ill defined and not encapsulated. Along with this, latter had more intense peri-tumoral desmoplastic reaction than the former.
In our cases, kidney could not be salvaged in both the patients but bowel, pancreas and major blood vessels namely, IVC, Aorta with celiac axis and superior mesenteric vessels were safely separated and it was fortunate that complete removal was possible with clear resection margins.
The primary goals of treatment with curative intent include achieving surgical resection with negative margins, reducing local recurrence, improving functional outcomes, and reducing the risk of distant metastasis.
Preoperative radiation is considered ineffective in reducing local recurrence and improving survival in retroperitoneal leiomyosarcoma. Tumor depth is an important prognostic factor independent of tumor size and histologic grade, directly correlating with a worse outcome.[21 22 23]
Conclusion
Retroperitoneal sarcomas are rare, rapidly growing tumors found in the 5th-6th decade of life. Early diagnosis and complete surgical resection offers the only hope of cure in them. Multi-disciplinary management helps in better management of such patients with optimal outcome.
References
- Neville A, Herts BR. CT characteristics of primary retroperitoneal neoplasms. Rev. Comput. Tomogr.2004; 45: 247–70.
- Rosenberg AE. WHO Classification of Soft Tissue and Bone, fourth edition: summary and commentary. Opin. Oncol.2013; 25: 571–3.
- Osman S, Lehnert BE, Elojeimy S et al. A comprehensive review of the retroperitoneal anatomy, neoplasms, and pattern of disease spread. Probl. Diagn. Radiol.2013; 42: 191–208.
- Fletcher CDM. WHO: in classification of tumours of soft tissue and bone. In: Bridge JA, Hogendoorn PCW, Mertens F, eds. World Health Orgn. 4th ed. WHO PRESS; 2013:468.
- Mentzel T, Schneider-Stock R. Lipogen differenzierte Tumoren. In: Klöppel G, Kreipe HH, Remmele W, eds. Pathologie (Kopf-Hals-Region, Weichgewebstumoren, Haut). 3rd ed. Springer; 2008:401-403.
- Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21: 271-281.
- Fanburg-Smith JC, Miettinen M. Liposarcoma with meningothelial-like whorls: a study of 17 cases of a distinctive histological pattern associated with dedifferentiated liposarcoma. Histopathology. 1998;33:414-424.
- Hasegawa T, Seki K, Hasegawa F, et al. Dedifferentiated liposarcoma of retroperitoneum and mesentery: varied growth patterns and histological grades–a clinicopathologic study of 32 cases. Hum Pathol. 2000;31:717-727.
- Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36:462-469.
- Kang Y, Horvai AE. P16 immunohistochemistry is less useful than MDM2 and CDK4 to distinguish dedifferentiated liposarcomas from other retroperitoneal mimics. Appl Immunohistochem Mol Morphol. 2017;25:58-63.
- Malkowicz SB. Retroperitoneal tumors: diagnosis, staging, surgery, management, and prognosis. Urologic oncology. 1st ed. Philadelphia: Saunders; 1997. p. 539-57.
- Swallow CJ, Strauss DC, Bonvalot S, Rutkowski P, Desai A, Gladdy RA, et al. Management of primary retroperitoneal sarcoma (RPS) in the adult: an updated consensus approach from the transatlantic australasian RPS working group. Ann Surg Oncol. (2021) 28(12):7873–88. doi: 10.1245/s10434-021-09654-z
- Gyorki D, Choong P, Slavin J, Henderson M. Importance of preoperative diagnosis for management of patients with suspected retroperitoneal sarcoma. ANZ J Surg. (2018) 88(4):274–7. doi: 10.1111/ans.14125
- Sogaard AS, Laurberg JM, Sorensen M, Sogaard OS, Wara P, Rasmussen P, et al. Intraabdominal and retroperitoneal soft-tissue sarcomas–outcome of surgical treatment in primary and recurrent tumors. World J Surg Oncol. (2010) 8:81. doi: 10.1186/1477-7819-8-81
- Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. (2005) 353(7):701–11. doi: 10.1056/NEJMra041866
- Hilal L, Barada K, Mukherji D, Temraz S, Shamseddine A. Gastrointestinal (GI) leiomyosarcoma (LMS) case series and review on diagnosis, management, and prognosis. Med Oncol. (2016) 33(2):20. doi: 10.1007/s12032-016-0730-3
- von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, et al. NCCN guidelines insights: soft tissue sarcoma, version 1.2021. J Natl Compr Canc Netw. (2020) 18(12):1604–12. doi: 10.6004/jnccn.2020.0058
- Swallow CJ, Strauss DC, Bonvalot S, Rutkowski P, Desai A, Gladdy RA, et al. Management of primary retroperitoneal sarcoma (RPS) in the adult: an updated consensus approach from the transatlantic australasian RPS working group. Ann Surg Oncol. (2021) 28(12):7873–88. doi: 10.1245/s10434-021-09654-z
- von Mehren M, Randall RL, Benjamin RS et al. Tissue sarcoma, Version 2.2018, NCCN clinical practice guidelines in oncology. Natl Compr. Cancer Netw.2018; 16: 536–63.
- Transatlantic Australasian Retroperitoneal Sarcoma Working G. Intercontinental collaborative experience with abdominal, retroperitoneal and pelvic schwannomas. J. Surg.2020; 107: 452–63.
- Cates JMM. The AJCC 8th Edition Staging System for Soft Tissue Sarcoma of the Extremities or Trunk: A Cohort Study of the SEER Database. J Natl Compr Canc Netw. 2018 Feb;16(2):144-152
- Maki RG, Moraco N, Antonescu CR, Hameed M, Pinkhasik A, Singer S, Brennan MF. Toward better soft tissue sarcoma staging: building on american joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol. 2013 Oct;20(11):3377-83.
- Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014 Sep;260(3):416-21; discussion 421-2.

Dr. Raghavendra Nagaraja
Consultant and Head – Surgical Gastroenterology

