Patient-Controlled Analgesia
Vaibhav V Savadi
Consultant Liver Transplant Anaesthesia, Kauvery Hospital, Chennai
Background
Although the term patient-controlled analgesia (PCA) covers a variety of techniques where patients self-administer analgesic drugs (e.g., entonox in labour or oral analgesia postoperatively), the term is usually taken to refer to self- administration of intravenous drugs. The technique was developed initially for the relief of pain in labour using a simple mechanical arrangement where patients opened a clamp to self-administer a dilute solution of meperidine. Sophisticated electronic pumps were subsequently developed but these were used mainly for research purposes. It was not until the late 1980s that simple, portable PCA pumps were introduced into every- day clinical practice. PCA is now used commonly in many hospitals. Although postoperative pain control is the commonest indication for PCA, the technique is also used in other acutely painful situations such as pancreatitis, rib fractures, sickle cell crisis and acute exacerbations of chronic pain.
The rationale for PCA
The marked between-patient and within-patient variability seen in the response to opioids in the postoperative period is one of the major limitations of the analgesic techniques that preceded PCA. Intermittent intramuscular opioid injections and fixed-rate infusions of opioids are unable to deal with a 10-fold difference in opioid consumption between patients in the postoperative period. Similarly, they are often not flexible enough to cope with the varying demands of an individual patient whose pain is likely to increase during mobilisation or physiotherapy or at different times of the day.
The underlying principle of PCA is that the patient controls the dose of analgesia administered. This allows each patient to receive the appropriate dose of analgesia for them at that particular time in their postoperative course. The pharmacokinetic basis of PCA is that patients titrate their plasma opioid concentrations to values above the minimum effective analgesic concentration (MEAC) but below the minimum toxic concentration (MTC), the so-called analgesic window (Fig. 1). Although this is a useful concept to explain PCA, it is important to remember that the log dose- response curve for opioid analgesia is not linear but a steep sigmoidal curve. This means that, once the analgesic threshold is reached, small increases in plasma concentration may result in effective analgesia. Further increases may lead to opioid side-effects rather than further improvements in analgesia. In some patients (e.g., elderly, obese patients undergoing upper abdominal surgery), the difference between MEAC and MTC may be so small that it will be very difficult to provide analgesia without significant opioid side-effects, including respiratory depression.
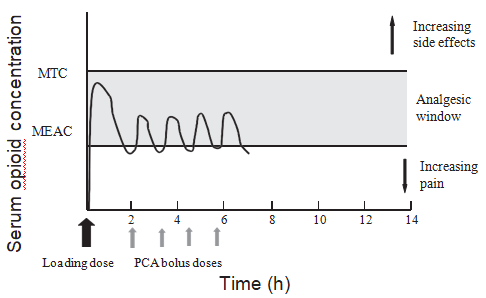
Fig. 1. Pharmacokinetic basis of patient-controlled analgesia
Key points
- Patient-controlled analgesia, when used appropriately, is a safe and effective method of providing opioid-based pain relief
- Safety and efficacy are optimized by education, local guidelines and monitoring supervised by an acute pain team
- Choice of opioid does not affect the incidence of side-effects or effectiveness of analgesia
- A background infusion increases the risk of respiratory depression
Table 1. Ideal characteristics of an electronic PCA device
| Regulatory approval |
| Ease of use for patients and staff and readily distinguishable from other infusion devices |
| Lightweight, portable, with a power source, battery back-up and a power failure alarm |
| Flexible programming to allow reliable delivery of a set dose lockout facility, maximum hourly dose background infusion, record of dose given and failed attempts, occlusion and air-in-the-line alarm |
| Method of telling the patient that a dose is available |
| Lock to prevent tampering |
Equipment
The majority of commercially available delivery systems are electronic pumps which are simplified, robust versions of the Cardiff Palliator that was introduced in 1976. The ideal characteristics of an electronic PCA device are shown in Table 1.
PCA should be administered to patients with colour-coded giving sets which incorporate anti-syphon and anti-reflux valves. Many of the ideal characteristics of a PCA pump are met by disposable devices which are able to provide PCA as effectively as electronic PCA pumps.
PCA regimens
PCA is a technique for the maintenance of analgesia. Therefore, it is assumed that the patient will have been titrated to comfort with a loading dose of opioid prior to the commencement of PCA. Variables of the PCA prescription are the choice of opioid drug and settings of the PCA pump, i.e. bolus dose, lockout interval, dose limits and background infusion.
Choice of drug
The most commonly used opioid for PCA is morphine but others are used also (e.g. diamorphine, meperidine, fentanyl, tramadol). There is no evidence from clinical studies that any particular opioid is more effective or associated with a lower incidence of opioid side-effects. However, individual patients may have a markedly different response to different opioid drugs. The benefits for an individual patient of changing from one opioid to another have to be weighed against the increased complexity and risks within a hospital of using more than one drug for PCA.
PCA settings
Bolus dose
The bolus dose delivered to the patient should be the smallest amount capable of producing an appreciable analgesic effect. If the bolus is too small to produce an effect, the patient can lose confidence in the system and will often stop pressing the demand button. If too large a bolus size is selected, the incidence of side-effects can be increased. Frequently prescribed bolus doses are morphine 1-2 mg, meperidine 10-20 mg and fentanyl 10-20 ïg. The volume of the bolus dose is also important. Volumes of < 0.5 ml will often fail to trigger an occlusion alarm.
Lockout interval
The lockout interval should be set to prevent further doses being delivered until the bolus dose has had time to achieve peak effect. Despite the fact that i.v. morphine takes 15 min to exert its peak effect, the lockout interval is usually set at 5-10 min.
Dose limits
Although most modern PCA pumps have the ‘safety feature’ of allowing the user to set hourly or 4-hourly limits for opioid usage, there is no clear opinion on how this facility should be used. It is not a substitute for careful patient monitoring to ensure patient safety.
Background infusion
There is little evidence that the use of a background infusion improves analgesia or sleep in the postoperative period. Furthermore, the addition of a background infusion has been shown to increase the incidence of respiratory depression and the incidence of programming errors compared with PCA alone. Although background infusions should not be used routinely, they may be useful in patients who are already on high dose opioids.
The widely used morphine PCA regimen of a 1 mg bolus dose with a 5 min lockout interval and no background infusion allows the patient to administer a maximum of 12 mg h-1. This maximum dose is adequate for the majority of patients in pain and ineffective analgesia is usually due to failure to use the device properly. Pre-operative patient education and post- operative re-inforcement are more likely to improve analgesia than merely increasing the bolus dose or introducing a back- ground infusion.
Patient factors
Patients’ expectations of quality of pain relief are likely to be formed by their previous experience and the information they receive pre-operatively. Quality of pain relief is not necessarily the most important factor for the patient when judging the quality of postoperative care. Several other factors are seen as important to patients, e.g. independence, self-control, privacy and re-assurance. The use of PCA for postoperative pain relief allows the patient to retain some degree of control at a time when they often have to relinquish other areas of personal control. Other advantages of PCA from the patient’s perspective include not having to bother nursing staff, avoidance of repeated intramuscular injections and a rapid onset of effective analgesia.
Patient selection
It is important to realize that not everybody will benefit from PCA. Any patient having an operation who is likely to require potent opioids for postoperative analgesia can be considered. Exclusions include patients unable to understand how to use the system, e.g. the very young, confusional states. The safe use of PCA in children over 5 years of age requires education of staff, patients and parents and the use of standardized protocols.
Although PCA has been shown to be more effective than intramuscular analgesia in elderly patients, caution should be exercised in this age group because of alterations in postoperative cognitive function and reduced opioid requirements. Care should be taken in patients with impaired renal excretion of the metabolites of morphine or meperidine and those who are particularly sensitive to the airway effects of opioids, e.g. morbid obesity, obstructive sleep apnoea.
Table 2. Risk factors for respiratory depression with PCA
| Background infusion |
| Morphine bolus dose > 1 mg |
| Elderly |
| Respiratory disease (including obstructive sleep apnoea) |
| Proxy control |
| Concomitant sedatives |
| Operator error |
| Equipment failure |
Safety of PCA
The major safety concern regarding the use of PCA is severe opioid-induced respiratory depression. The incidence of this complication in hospitals with acute pain services (APS) is 0.1-0.8%. This compares with a risk of 0.2-0.9% for respiratory depression following intermittent intramuscular opioid analgesia and 1.7% for continuous i.v. opioid infusions.
However, the risk of respiratory depression with PCA is increased to 1.1-3.9% if a background infusion is used. Although the incidence of respiratory depression in a hospital with an APS and appropriate supervision is likely to be low, well-publicized cases of respiratory depression still occur. Table 2 lists the risk factors for the development of respiratory depression.x
Documented operator errors have included the use of the wrong drug (e.g. mixtures designed for epidural use) and programming errors (e.g. inappropriate bolus doses), incorrect drug concentrations and incorrect background infusions.
Equipment failure is a rare cause of respiratory depression with PCA as modern pumps have several ‘fail-safe’ design features. Early reports of siphoning of syringe contents associated with pump failure or cracked syringes have led to the routine use of anti-syphon valves and avoidance of positioning PCA pumps above patient heart level. Failure of anti- reflux valves or failure to use them has led to cases of respiratory depression.
The safe use of PCA within a hospital relies on the implementation of agreed guidelines and supervision by the APS (Table 3). There is clear evidence that this approach improves the safety and efficacy of PCA.
Table 3. Key responsibilities of the acute pain service (APS)
| Provision of a continuing education programme for both medical and nursing staff caring for patients using PCA |
| Ensuring the use of a single regimen throughout a hospital so all staff are familiar with the drug, pump and settings |
| Clear instructions on who is responsible for problems related to PCA and provision of easy access to the APS if they arise |
| Ensuring availability of staff capable of dealing with emergencies associated with PCA, e.g. respiratory arrest |
| Ensuring minimum standards of observations, including respiratory rate, sedation score, pain score at rest and on movement, pulse and blood pressure |
| Provision of a written action plan for the management of common problems, e.g. inadequate analgesia, emesis, respiratory depression. |
Patient outcome
Have the initial claims for PCA in terms of improved analgesia, reduced opioid consumption, high levels of patient satisfaction, improved outcome and a reduction in side-effects been substantiated by recent clinical studies?
Analgesic efficacy
In the majority of studies, PCA has been shown to be as effective or more effective than intermittent intramuscular analgesia. The inability to demonstrate a conclusive improvement in analgesic efficacy is not as surprising as it seems as many of the studies compare PCA with optimised forms of intramuscular analgesia in well-staffed units. A reduction in opioid consumption with PCA compared with other forms of opioid analgesia has not been confirmed in clinical trials.
Patient satisfaction
The reports of high levels of patient satisfaction due to improved patient-control have recently been tempered by reports that patients perceive several disadvantages to using PCA. In a study where a third of patients feared an overdose or addiction, only 43% of patients had received pre-operative education. Use of written information at an early stage may be useful to allay patient anxiety.
Postoperative morbidity
In terms of surgical outcome, there is little evidence to suggest that PCA is associated with a more rapid recovery of bowel and respiratory function or a reduction in hospital stay.
Side-effects
A variety of drugs have been used to treat the side-effects of opioids administered by PCA either symptomatically (e.g. anti- emetics), or indirectly by improving the efficacy of PCA with opioid-sparing drugs (e.g. NSAIDs and ketamine). Postoperative nausea and vomiting (PONV) are a common complaint in the post- operative period and is often regarded by patients (and staff) as more troublesome than inadequate analgesia. Although PONV has not been shown to be more common in patients using PCA, failure to treat PONV in these patients often results in abandonment of PCA resulting in poor analgesia.
There is little consensus on the ideal drug, or combination of drugs, that can be best utilized to prevent PONV. Various strategies have been employed and none are entirely successful. Droperidol, cyclizine and ondansetron have all been added to PCA syringes. There is still no clear evidence that the routine addition of anti-emetics to PCA syringes is more beneficial than separate administration of anti-emetics. Any potential benefits have to be offset against the side-effects and increased complexity and cost of adding anti-emetics to PCA syringes.
Conclusions
PCA has become established in the last decade as a safe and effective method of pain relief. The degree of safety and effi- cacy has been clearly shown to increase if the technique is supervised by an APS. Whilst there is little evidence that PCA has marked advantages over well-organised intermittent intra- muscular analgesia, the latter technique is more difficult to provide on busy, poorly-staffed wards.
Future developments in PCA include the use of different routes of administration (e.g. intranasal route), new drug delivery systems (e.g. iontophoresis) and new pump technology (e.g. adaptive PCA).
References
- Ballantyne JC, et al. Postoperative patient-controlled analgesia: meta-analyses of initial randomised control trials. J Clin Anaesth. 1993;5:182-93.
- Chumbley GM, et al. Patient-controlled analgesia: an assessment by 200 patients. Anaesth. 1998;53:216-21.
- Coleman SA, et al. Audit of postoperative pain control. Influence of a dedicated pain nurse. Anaesth. 1996;51:1093-6.
- Etches RC. Patient-controlled analgesia. Surg Clin North Am 1999;79:297-312.
- Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36-46.
