Journal Scan
Journal scan: A review of 10 recent papers of immediate clinical significance, harvested from major international journals
From the desk of the Editor-in-Chief
(1). Mahase E, et al. COVID-19: UK becomes first country to authorise antiviral molnupiravir. BMJ. 2021;375:n2697.
The UK’s medicines regulator has issued temporary authorisation of the antiviral drug molnupiravir for the treatment of mild to moderate COVID-19 in adults with at least one risk factor for severe illness.
The Medicines and Healthcare Products Regulatory Agency is the first regulator in the world to approve the drug, of which the UK has ordered 480000 courses.
Molnupiravir will be rolled out to the people most at risk of COVID-19, with the aim of reducing symptom severity and easing pressure on the NHS over winter, as per the Department of Health and Social Care for England.
Interim phase III trial results, released through a press release by the drug’s manufacturer, MSD, found that molnupiravir reduced the risk of admission to hospital or death by around 50% in non-hospitalised adults who had mild to moderate COVID-19 and were at risk of poor outcomes.
The release said that 7.3% of patients (28 of 385) who received molnupiravir and 14.1% of those taking placebo (53 of 377) either were admitted to hospital or had died by day 29 after randomisation. At day 29 no deaths were reported in the molnupiravir group, while eight were reported in the placebo group. Recruitment to the trial was then stopped on the advice of the independent data monitoring committee because of the positive results.
The US has ordered 1.7 million courses of molnupiravir, and MSD said it also had plans to ensure that the treatment could be accessed by low and middle income countries. This includes a tiered pricing approach, based on World Bank country income criteria, and non-exclusive voluntary licensing agreements with established manufacturers of generic drugs to accelerate availability in more than 100 low and middle income countries.
(2). Tanne JH, et al. COVID-19: US doctors begin administering vaccine to children aged 5-11. BMJ. 2021;375:n2693.
US physicians began giving the Pfizer BioNTech vaccine to children aged 5-11 on 3 November, immediately after the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) authorised its use.
There are about 28 million children in that age group in the US. They will receive a 10 µg dose (about one third the adult dose) of the vaccine followed by a second jab three weeks later. Previously, vaccination had been approved only for children over 12.
The FDA had previously authorised the Pfizer BioNTech vaccine for children aged 5-11 and the CDC’s advisory committee on immunisation practices recommended the child dose on 2 November. CDC director Rochelle Walensky immediately approved it, saying, “We have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated. As a mom, I encourage parents with questions to talk to their paediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated.”
Gerald Harmon, president of the American Medical Association, said the vaccine authorisation was “a critical step toward protecting this population from COVID-19 infections. There is overwhelming evidence showing that COVID-19 vaccines are safe and effective but we know many parents and families will have questions. We encourage parents to speak with their child’s physician and review trusted resources, such as getvaccineanswers.org, to get the information they need to make an informed decision.”
The American Academy of Paediatrics also supported the CDC’s decision, saying that it “will protect children’s health and allow them to fully engage in all the activities that are so important to their health and development.” It said children who had had a COVID-19 infection should receive the vaccine “to prevent a second infection and to lower the risk of severe illness and hospital admission.” It suggested children receive the COVID-19 vaccine and the flu vaccine during a single visit to a doctor or clinic. But it also said that mask wearing should continue.
(3). Pope MK, et al. Cardioversion in patients with newly diagnosed non-valvular atrial fibrillation: observational study using prospectively collected registry data. BMJ 2021;375:e066450.
Objective: To investigate the clinical outcomes of patients who underwent cardioversion compared with those who did not have cardioverson in a large dataset of patients with recent onset non-valvular atrial fibrillation.
Design: Observational study using prospectively collected registry data (Global Anticoagulant Registry in the FIELD-AF-GARFIELD-AF).
Setting: 1317 participating sites in 35 countries.
Participants: 52057 patients aged 18 years and older with newly diagnosed atrial fibrillation (up to six weeks’ duration) and at least one investigator determined stroke risk factor.
Main outcome measures: Comparisons were made between patients who received cardioversion and those who had no cardioversion at baseline, and between patients who received direct current cardioversion and those who had pharmacological cardioversion. Overlap propensity weighting with Cox proportional hazards models was used to evaluate the effect of cardioversion on clinical endpoints (all cause mortality, non-haemorrhagic stroke or systemic embolism, and major bleeding), adjusting for baseline risk and patient selection.
Results: 44 201 patients were included in the analysis comparing cardioversion and no cardioversion, and of these, 6595 (14.9%) underwent cardioversion at baseline. The propensity score weighted hazard ratio for all cause mortality in the cardioversion group was 0.74 (95% confidence interval 0.63 to 0.86) from baseline to one year follow-up and 0.77 (0.64 to 0.93) from one year to two year follow-up. Of the 6595 patients who had cardioversion at baseline, 299 had a follow-up cardioversion more than 48 days after enrolment. 7175 patients were assessed in the analysis comparing type of cardioversion: 2427 (33.8%) received pharmacological cardioversion and 4748 (66.2%) had direct current cardioversion. During one year follow-up, event rates (per 100 patient years) for all cause mortality in patients who received direct current and pharmacological cardioversion were 1.36 (1.13 to 1.64) and 1.70 (1.35 to 2.14), respectively.
Conclusion: In this large dataset of patients with recent onset non-valvular atrial fibrillation, a small proportion were treated with cardioversion. Direct current cardioversion was performed twice as often as pharmacological cardioversion, and there appeared to be no major difference in outcome events for these two cardioversion modalities. For the overall cardioversion group, after adjustments for confounders, a significantly lower risk of mortality was found in patients who received early cardioversion compared with those who did not receive early cardioversion.
(4). Biccard BM, et al. Prevention of surgical site infection in low-resource settings. Lancet 2021;398(10312);P1664-1665.
WHO defines universal health coverage (UHC) as “access to needed essential health services, without financial hardship”. UHC requires about US$100 per head for an essential package of 218 interventions, and approximately $50 per head for a basic package of 108 of the highest priority interventions. Yet, estimates in 2016 suggested that only nine of the 49 low-income and middle-income countries (LMICs) could afford the essential package, and that 16 countries could afford the 108 highest priority interventions of the basic package. At an average government spend on health in low-income countries of only $9 per head in 2018 (1.2% of Gross National Product), literally every cent counts.
Surgical care is an indivisible component of UHC,4 yet the outcomes in low-income countries are poor. Provision of quality surgical care in these countries is difficult because resources and finances are limited. International guidelines that are blind to these barriers can unwittingly compromise quality care elsewhere when scarce financial resources are wasted to comply with guidelines that have a poor evidence base. Availability of a stable surgical supply chain is a challenge for low-income countries, especially because production is often international and procurement is expensive. The resultant costs of surgical supplies are commonly transferred to the patients, often leading to catastrophic out-of-pocket expenditure. Those who cannot afford these costs often do not receive surgical care. To realise the aspiration of UHC, affordable, sustainable solutions to the surgical needs of patients are needed.
Reported in The Lancet, the FALCON trial 8 is an important surgical trial for advancing UHC in low-income settings. Surgical site infections (SSI) predominate perioperative complications, with a higher burden and more antibiotic resistance in low-income countries.
The need for appropriate global guidelines for prevention of SSI is therefore important. However, some recommendations are based on little evidence, with a negative financial effect in low-income countries.
The FALCON trial provides the evidence necessary to inform the appropriateness of the WHO recommendation of 2% alcoholic chlorhexidine skin preparation and triclosan-coated sutures to prevent SSI in abdominal surgery in LMICs. Before this study, the evidence was generally weak, with little data from LMICs to support such a recommendation.
The FALCON trial found that neither 2% alcoholic chlorhexidine skin preparation nor triclosan-coated sutures provided benefit when compared with povidone-iodine skin preparation and non-coated sutures. The implications of these findings are that cheaper skin preparations and sutures can be safely used in low-resource environments with equivalent efficacy to prevent SSI, freeing up funds to improve the quality of care elsewhere.
The FALCON trial provides robust data for the prevention of SSI in LMICs. The trial included 5788 participants (61% were female), from 54 hospitals in seven LMICs, and included children (14%) and those needing emergency surgery (67%). The trial is powered at 90% for both clean-contaminated and contaminated or dirty surgery, which is important because of potentially differing SSIs associated with exposure and non-exposure to bowel contents. There are numerous appropriate sensitivity analyses that are all consistent with the primary outcome of the trial. It is therefore likely that the findings are generalisable to patients from LMICs having abdominal surgery. There are some trial limitations, including unmasked theatre staff, and the introduction of telephonic follow-ups during the COVID-19 pandemic. However, we believe that these limitations have not significantly compromised the trial findings to detract from the robust trial signal.
Similar to FALCON, evidence is needed to challenge other controversial practice guidelines in low-income countries, such as the WHO recommendation for the use of liberal inspired oxygen concentrations of 80% during surgery to prevent SSI. The retraction of several trials has raised further concerns regarding the evidence base. The COVID-19 pandemic has highlighted the importance of oxygen as a scarce health-care resource, emphasising the need to establish the evidence for the recommendation for liberal inspired oxygen to prevent SSI. This issue is being addressed in another large pragmatic trial (PErioperative respiratory care and outcomes for patieNts Undergoing high risk abdominal surgery [PENGUIN], NCT04256798) also led by the NIHR Global Health Research Unit on Global Surgery.
(5). Pinato DJ, et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCOVID retrospective, multicentre registry study. 2021.
Background
The medium-term and long-term impact of COVID-19 in patients with cancer is not yet known. In this study, we aimed to describe the prevalence of COVID-19 sequelae and their impact on the survival of patients with cancer. We also aimed to describe patterns of resumption and modifications of systemic anti-cancer therapy following recovery from SARS-CoV-2 infection.
Methods
OnCOVID is an active European registry study enrolling consecutive patients aged 18 years or older with a history of solid or haematological malignancy and who had a diagnosis of RT-PCR confirmed SARS-CoV-2 infection. For this retrospective study, patients were enrolled from 35 institutions across Belgium, France, Germany, Italy, Spain, and the UK. Patients who were diagnosed with SARS-CoV-2 infection between Feb 27, 2020, and Feb 14, 2021, and entered into the registry at the point of data lock (March 1, 2021), were eligible for analysis. The present analysis was focused on COVID-19 survivors who underwent clinical reassessment at each participating institution. We documented prevalence of COVID-19 sequelae and described factors associated with their development and their association with post-COVID-19 survival, which was defined as the interval from post-COVID-19 reassessment to the patients’ death or last follow-up. We also evaluated resumption of systemic anti-cancer therapy in patients treated within 4 weeks of COVID-19 diagnosis. The OnCOVID study is registered in ClinicalTrials.gov, NCT04393974.
Findings
2795 patients diagnosed with SARS-CoV-2 infection between Feb 27, 2020, and Feb 14, 2021, were entered into the study by the time of the data lock on March 1, 2021. After the exclusion of ineligible patients, the final study population consisted of 2634 patients. 1557 COVID-19 survivors underwent a formal clinical reassessment after a median of 22.1 months (IQR 8.4-57.8) from cancer diagnosis and 44 days (28-329) from COVID-19 diagnosis. 234 (15.0%) patients reported COVID-19 sequelae, including respiratory symptoms (116 [49.6%]) and residual fatigue (96 [41.0%]). Sequelae were more common in men (vs women; p=0.041), patients aged 65 years or older (vs other age groups; p=0.048), patients with two or more comorbidities (vs one or none; p=0.0006), and patients with a history of smoking (vs no smoking history; p=0.0004). Sequelae were associated with hospitalisation for COVID-19 (p<0.0001), complicated COVID-19 (p<0.0001), and COVID-19 therapy (p=0.0002). With a median post-COVID-19 follow-up of 128 days (95% CI 113-148), COVID-19 sequelae were associated with an increased risk of death (hazard ratio [HR] 1.80 [95% CI 1.18-2.75]) after adjusting for time to post-COVID-19 reassessment, sex, age, comorbidity burden, tumour characteristics, anticancer therapy, and COVID-19 severity. Among 466 patients on systemic anti-cancer therapy, 70 (15.0%) permanently discontinued therapy, and 178 (38.2%) resumed treatment with a dose or regimen adjustment. Permanent treatment discontinuations were independently associated with an increased risk of death (HR 3.53 [95% CI 1.45-8.59]), but dose or regimen adjustments were not (0.84 [0.35.2.02]).
Interpretation
Sequelae post-COVID-19 affect up to 15% of patients with cancer and adversely affect survival and oncological outcomes after recovery. Adjustments to systemic anti-cancer therapy can be safely pursued in treatment-eligible patients.
(6). The Lancet Commission on health futures 2030: growing up in a digital world. 2021.
https://www.thelancet.com/commissions/governing-health-futures-2030.
Executive Summary
Digital transformations are well underway in all areas of life. These have brought about substantial and wide-reaching changes, in many areas, including health. But large gaps remain in our understanding of the interface between digital technologies and health, particularly for young people.
The Lancet and Financial Times Commission on governing health futures 2030: growing up in a digital world argues digital transformations should be considered as a key determinant of health. But the Commission also presses for a radical rethink on digital technologies, highlighting that without a precautionary, mission-oriented, and value-based approach to its governance, digital transformations will fail to bring about improvements in health for all.
(7). Can digital technologies improve health? Lancet. 2021;398(10312):P1663.
If you have followed the news on digital technology and health in recent months, you will have read of a blockbuster fraud trial centred on a dubious blood-testing device, a controversial partnership between a telehealth company and a data analytics company, a social media company promising action to curb the spread of vaccine misinformation, and another addressing its role in the deteriorating mental health of young women. For proponents and critics alike, these stories encapsulate the health impact of many digital technologies, and the uncertain and often unsubstantiated position of digital technologies for health. The Lancet and Financial Times Commission on governing health futures 2030: growing up in a digital world, brings together diverse, independent experts to ask if this narrative can still be turned around? Can digital technologies deliver health benefits for all?
Digital technologies could improve health in many ways. For example, electronic health records can support clinical trials and provide large-scale observational data. These approaches have underpinned several high-profile research findings during the COVID-19 pandemic. Sequencing and genomics have been used to understand SARS-CoV-2 transmission and evolution. There is vast promise in digital technology, but the Commission argues that, overall, digital transformations will not deliver health benefits for all without fundamental and revolutionary realignment.
Globally, digital transformations are well underway and have had both direct and indirect health consequences. Direct effects can occur through, for example, the promotion of health information or propagating misinformation. Indirect ones can happen via effects on other determinants of health, including social, economic, commercial, and environmental factors, such as influencing people’s exposure to marketing or political messaging. Children and adolescents growing up in this digital world experience the extremes of digital access. Young people who spend large parts of their lives online may be protected or vulnerable to online harm. But many individuals remain digitally excluded, affecting their access to education and health information. Digital access, and the quality of that access, must be recognised as a key determinant of health. The Commission calls for connectivity to be recognised as a public good and human right.
Describing the accumulation of data and power by dominant actors, many of which are commercial, the Commissioners criticise business models based on the extraction of personal data, and those that benefit from the viral spread of misinformation. To redirect digital technologies to advance universal health coverage, the Commission invokes the guiding principles of democracy, equity, solidarity, inclusion, and human rights. Governments must protect individuals from emerging threats to their health, including bias, discrimination, and online harm to children. The Commission also calls for accountability and transparency in digital transformations, and for the governance of misinformation in health care-basic principles, but ones that have been overridden in a quest for freedom of expression and by the fear that innovation could be sidelined. Public participation and codesign of digital technologies, particularly including young people and those from affected communities, are fundamental.
The Commission also advocates for data solidarity, a radical new approach to health data in which both personal and collective interests and responsibilities are balanced. Rather than data being regarded as something to be owned or hoarded, it emphasises the social and relational nature of health data. Countries should develop data trusts that unlock potential health benefits in public data, while also safeguarding it.
Digital transformations cannot be reversed. But they must be rethought and changed. At its heart, this Commission is both an exposition of the health harms of digital technologies as they function now, and an optimistic vision of the potential alternatives. Calling for investigation and expansion of digital health technologies is not misplaced techno-optimism, but a serious opportunity to drive much needed change. Without new approaches, the world will not achieve the 2030 Sustainable Development Goals.
However, no amount of technical innovation or research will bring equitable health benefits from digital technologies without a fundamental redistribution of power and agency, achievable only through appropriate governance. There is a desperate need to reclaim digital technologies for the good of societies. Our future health depends on it.
(8). Dawson Q. Anurag Agrawal: harnessing digital technologies for better health. Lancet. 2021;398(10312):P1678.
Growing up in a family of scientists, Anurag Agrawal recalls that research was a frequent topic of conversation in his youth. But it was the launch of the first IBM computer in 1981, when he was 9 years old, that “left a lasting impression and instilled a personal lifelong interest in computation”, he says. Now a physician, researcher, and the Director of the CSIR Institute of Genomics and Integrative Biology (CSIR-IGIB) in New Delhi, India, he has brought these passions together in his role as a Co-Chair of the Lancet and Financial Times Commission on governing health futures 2030: growing up in a digital world. He describes the vision for the Commission’s role as “undercutting the hype, retaining the positivity about the future impact of this area, whilst stressing the need to re-establish solid fundamentals for a better future”.
Agrawal’s interest in digital health and data was evident at the outset of his medical education. Before studying medicine in the early 1990s at the All India Institute of Medical Sciences, Delhi, he says he always intended to “carry on informal training in computer science”. Agrawal recalls how “I was never one who enjoyed clinical outpatient interactions, I liked numbers and data more than usual medicine and I knew I wanted to continue in this pathway.” During medical studies, Agrawal worked alongside his father, who was a respiratory physiologist, to “invent a new way of measuring lung volumes using a body-box, eliminating the need for the patient to pant against a closed shutter”, he says. This project was his first entry into academic publishing and also crystallised his decision to pursue further research in respiratory physiology, an area which was “interesting in terms of mathematics and engineering, and thus amenable to the type of computation work I was doing”, he says.
Moving to Baylor College of Medicine in Houston, TX, USA, in July, 1996, first as a medical resident in internal medicine and then subsequently as a research fellow, Agrawal initially intended to pursue clinical studies in respiratory physiology and critical care under Joseph Rodarte. But when his supervisor became ill Agrawal completed his training at Baylor with the pulmonologist Burton Dickey. It was at this transition point from a respiratory physiologist to a respiratory cellular and molecular biologist when Agrawal discovered “that pulmonary physiology was a field that had stopped growing, whilst molecular biology and genomics was rapidly expanding”, he says.
Yet when he looked ahead at a career path in the USA, Agrawal thought it was likely to require a focus on an increasingly narrow field of inquiry, and heeding calls from his friend and mentor Samir Brahmachari, the Founder Director of the CSIR-IGIB, he decided to return to India. Agrawal describes this move as “a complex decision but rather perfectly timed”. After completing a PhD in physiology at the University of Delhi in 2007, he joined CSIR-IGIB as a senior scientist and was tasked with setting up a Center of Excellence for Translational Research in Asthma and Lung Disease. “The real advantage of India, over the US, is its people-based funding, in addition to project-based funding, allowing flexible opportunities to maintain a wider career pathway”, Agrawal says. The emerging field of digital health also had an impact on Agrawal’s outlook at this time. On their return to India, his wife, Anjali Agrawal, established a teleradiology company and it was through her practice that Agrawal “saw the entire evolution of digital health and how it changes medical practice first-hand”, he says.
At CSIR-IGIB, he moved up the ranks, becoming Director in 2017. Agrawal’s focus at IGIB is the ‘multi-scale understanding of respiratory health, from cell biology to population studies. Beyond respiratory research, my interests are in policies and frameworks for deployment and use of emerging technologies such as genomics or artificial intelligence towards a better future”, he says. Agrawal talks of his research interests being driven by curiosity and “what we know the least and what we talk about the most”. As he explains, “Currently, the field of applications of computers, data science, artificial intelligence to health care is an area where I realised there was a lot of superficiality to it and more needed to be done. I do tend to gravitate towards such areas, where foundations are being laid.” Commenting on the broader digital health context in India, he describes how digital foundations are expanding with the announcement on Sept 27, 2021, of the national health ID system and the estimated 700 million people in India who now have a digital ID. “Impact from my point of view is now greatest in the area of precision health and big data, which also suit my natural interests’, he says.
As Director of the CSIR-IGIB, one of the CSIR’s 37 national laboratories across India, Agrawal emphasises his priority now is about ‘finding more younger people who want to build careers in intersectoral pathways around this area, because everything takes collaborative effort”. “Digital health, informatics, genomics is one such combination. It could equally be medicine and genomics”, he explains. Setting out his vision for the future, Agrawal comments: “Systems of old labels will always impede medicine since many labels are outdated or incorrect. We thus need to create a new vision of health which is different, which is not about using computers to only recapitulate our semi-correct labels. This cannot happen without huge amounts of data across different periods, lifespans, and diseases, and thus the foundations of this new endeavour must begin in this new digital age.”
(9). Taquet M, et al. Depression and anxiety disorders during the COVID-19 pandemic: knowns and unknowns. Lancet. 2021;398(10312):P1665-1666.
The COVID-19 pandemic has taken a toll on people’s mental health. Yet, the global extent of this impact remains largely unknown. By leveraging the best available data from surveys around the world with measurements of anxiety and depression both before and during the pandemic, and analysing these data using the Global Burden of Disease Study (GBD) model, the COVID-19 Mental Disorders Collaborators provide global insight into the burden of depression and anxiety disorders during the pandemic to date. The authors estimated a significant increase in the prevalence of both major depressive disorder (with an estimated additional 53.2 million [95% uncertainty interval 44.8-62.9] cases worldwide-ie, a 27.6% [25.1-30.3] increase) and anxiety disorders (76.2 million [64.3-90.6] additional cases-ie, a 25.6% [23.2-28.0] increase) since before the pandemic. Increased prevalence was seen for both males and females across the lifespan. These findings are all the more concerning because depressive and anxiety disorders were already leading causes of disability worldwide.
The study has unique strengths. First, by using the GBD model, it translates crude estimates from heterogeneous surveys into numbers of additional cases and disability-adjusted life-years. This makes the findings more tangible for policy makers, academics, charities, and the general public. Second, the study leverages data on COVID-19 impact indicators (i.e., human mobility, SARS-CoV-2 infection rates, and excess mortality). The COVID-19 Mental Disorders Collaborators estimated these indicators for all countries and territories and used them to inform the extrapolation of changes in prevalence to countries with no available survey data. Furthermore, the authors assessed the generalisability of their estimates for countries with no available surveys using a leave-one-country-out cross-validation approach, in which changes in prevalence were estimated using the GBD model as if survey data for one country were not available and the prediction was then compared with the actual survey data.
The study also has a few key limitations, largely resulting from the available data rather than the approach used to analyse them. First, direct measurements on changes in prevalence of depressive and anxiety disorders are not available in large regions of the world (eg, within South America and Africa). For these regions, the GBD model has to extrapolate estimates from other regions (e.g., the USA or Europe), which are very different on many levels (economically, demographically, politically, and culturally). This extrapolation might be unreliable, as shown in the cross-validation results. For instance, the GBD model predicts a substantial increase in the prevalence of major depressive disorder in Denmark and almost no change in China whereas the opposite is observed in surveys. Second, most of the available data are based on self-report scales (e.g., Patient Health Questionnaire-9 or General Anxiety Disorder-7 [GAD-7]), which measure symptoms rather than actual diagnoses. Although both symptoms and diagnoses are important, the difference between them is relevant in the pandemic context. A diagnosis of anxiety disorder according to the tenth International Classification of Diseases and Related Health Problems requires that individuals recognise their emotional distress as excessive or unreasonable. GAD-7 does not capture this aspect. For an individual at high risk of COVID-19 complications to constantly feel nervous and afraid would not be unreasonable (hence, not meeting requirements for an anxiety disorder), yet it would yield a high GAD-7 score. Finally, the study is unable to identify what is causing the increased burden of major depressive disorder and anxiety. In particular, the relative contributions to the prevalence of depression and anxiety disorders of direct consequences of COVID-19 illness, some measures used to curb the propagation of the virus (e.g., lockdowns), and other correlates of the pandemic (e.g., economic austerity) remain elusive.
By synthesising the best available data, this study not only reveals what we do know, but also-crucially-exposes what we still do not know. These known unknowns have implications for interpretation of the findings. The paucity of direct measurements in most countries implies that the findings are unable to inform on specific countries that have been more affected than others. Aid programmes aimed at improving population mental health are clearly needed widely, and this study is unable to suggest specific countries to be targeted first. Measurement of clinical diagnoses will be needed to plan service provision, ascertain the burden of the pandemic in terms of mental disorders, and forecast social and economic consequences. Crucially, identifying causal mechanisms, and modifiable mechanisms in particular, will be important to design and deliver the right interventions to the right people.
The findings of this study should urgently incentivise more research to determine the fuller geographical distribution of depression and anxiety disorders, the prevalence of depressive and anxiety disorders, and the underpinning mechanisms to improve mental health in the context of the COVID-19 pandemic globally.
(10). Di Maida F, et al. A high prostatic-specific antigen with a large pelvic mass indicates a prostatic cystadenocarcinoma. Lancet. 2021.
A 63-year-old man presented to our emergency department with a 1-year history of severe nocturia and straining to pass urine. Additionally, he reported having tenesmus and abdominal pain in the 2 weeks prior to attending. The patient had no significant medical history.
On examination we found his abdomen to be moderately distended; it was soft and normal bowels sounds could be heard. A rectal examination showed a large pelvic mass with an irregular surface occupying the whole rectal ampulla.
Laboratory investigations found a haemoglobin concentration of 11.8 g/dL (normal 14.0-18.0) and a white blood cell count of 5.41 x 109 per L (normal 4.0-10.0). The patient had a serum creatinine concentration of 1.37 mg/dL (normal 0.80-1.30), a blood sodium concentration of 139 mEq/L (normal 135-145) and a negative urinalysis; the prostate-specific antigen (PSA) serum concentration was 144 ng/mL (normal <4.0).
Multiparametric prostatic MRI showed an 18 cm x 13 cm lesion in the pelvis-made up of numerous, pluri-lobulated cystic areas with a heterogeneous signal; with T2 hyperintensity in some loci-indicating fluid, and T2 hypointensity in other parts-suggestive of intracystic bleeding. The cystic lesions were in the basal and middle portions of the prostate gland, which were completely unstructured and recognisable, only in part, in the mid-apical and anterior portions. No significant lymphadenopathy was seen (figure). A bone scan showed no evidence of metastatic spread. Initially, we considered the mass to be a giant prostatic cystadenoma and a three-dimensional reconstruction model was obtained to choose the most appropriate surgical strategy (figure).
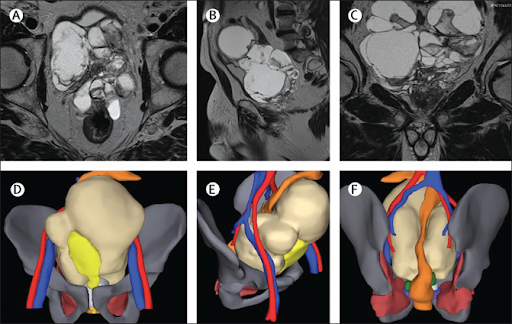
We carried out an open, radical prostatectomy using combined retrograde and anterograde approaches with limited pelvic lymph node dissection. The bladder was completely dislocated anteriorly and ended up in the right portion of the pelvis; it showed no signs of infiltration. Fluid obtained from aspiration of the cysts, sent for extemporaneous cytological analysis, had no neoplastic cells. The final histopathological examination showed a giant multilocular cystadenocarcinoma: ductal and acinar adenocarcinoma (Gleason score 4 + 4 = 8) mixed with the cystic tumour. Two 1 mm positive surgical margins-one apical and one posterolateral on the right side-were recorded on the prostate.
The tumour stage was pT3bN0 with nine lymph nodes, which when removed showed no tumour involvement. High-power microscopy showed nuclear stratifications, papillary structures, and Roman arch formations in the cystically dilated glands. The first postoperative PSA serum concentration was 0.12 ng/mL.
Because of the aggressive characteristics of the tumour and the adverse risk factors for early biochemical failure-the tumour stage, histology, and high PSA level-we decided to treat the prostate bed with adjuvant radiotherapy together with long-term androgen deprivation therapy.
At 6 month follow-up, the PSA serum concentration was undetectable and the patient had reached a satisfactory continence status using one pad per day.
Prostate cystadenocarcinoma is a very rare malignancy sometimes presenting as a large tumour that may be difficult to diagnose; its cystic nature means that it is not always possible to obtain a preoperative cytological or histopathological confirmation of the diagnosis that might then guide surgical management. Differentiating it from other pelvic cystic masses-including its benign counterpart, prostatic cystadenoma-is key. A particularly high PSA serum concentration-as seen in our patient-remains, we believe, the most valuable indicator of malignancy, even if preoperative imaging shows no metastatic spread and intraoperative cytological analysis does not indicate a tumour.
