Journal Scan
(1). Shi T, et al. Risk of serious COVID-19 outcomes among adults with asthma in Scotland: a national incident cohort study. Lancet Respir Med. 2022;S2213-2600(21)00543-9.
Background
There is considerable uncertainty over whether adults with asthma should be offered booster vaccines against SARS-CoV-2 and, if so, who should be prioritised for booster vaccination. We were asked by the UK’s Joint Commission on Vaccination and Immunisation to undertake an urgent analysis to identify which adults with asthma were at an increased risk of serious COVID-19 outcomes to inform deliberations on booster COVID-19 vaccines.
Interpretation
Adults with asthma who have required two or more courses of oral corticosteroids in the previous 2 years or a hospital admission for asthma before March 1, 2020, are at increased risk of both COVID-19 hospitalisation and ICU admission or death. Patients with a recent asthma attack should be considered a priority group for booster COVID-19 vaccines.
(2). Shi Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet. 2022;399(10321):259-69.
Background
Pharmacotherapy provides an option for adults with overweight and obesity to reduce their bodyweight if lifestyle modifications fail. We summarised the latest evidence for the benefits and harms of weight-lowering drugs.
Interpretation
In adults with overweight and obesity, phentermine-topiramate and GLP-1 receptor agonists proved the best drugs in reducing weight; of the GLP-1 agonists, semaglutide might be the most effective.
(3). Jovin TG, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet 2022;399(10321):P249-58.
Background
Trials examining the benefit of thrombectomy in anterior circulation proximal large vessel occlusion stroke have enrolled patients considered to have salvageable brain tissue, who were randomly assigned beyond 6 h and (depending on study protocol) up to 24 h from time last seen well. We aimed to estimate the benefit of thrombectomy overall and in prespecified subgroups through individual patient data meta-analysis.
Methods
We did a systematic review and individual patient data meta-analysis between Jan 1, 2010, and March 1, 2021, of randomised controlled trials of endovascular stroke therapy. In the Analysis Of Pooled Data From Randomized Studies Of Thrombectomy More Than 6 Hours After Last Known Well (AURORA) collaboration, the primary outcome was disability on the modified Rankin Scale (mRS) at 90 days, analysed by ordinal logistic regression. Key safety outcomes were symptomatic intracerebral haemorrhage and mortality within 90 days.
Findings
Patient level data from 505 individuals (n = 266 intervention, n = 239 control; mean age 68.6 years [SD 13.7], 259 [51.3%] women) were included from six trials that met inclusion criteria of 17 screened published randomised trials. Primary outcome analysis showed a benefit of thrombectomy with an unadjusted common odds ratio (OR) of 2.42 (95% CI 1.76-3.33; p < 0.0001) and an adjusted common OR (for age, gender, baseline stroke severity, extent of infarction on baseline head CT, and time from onset to random assignment) of 2.54 (1.83-3.54; p < 0.0001). Thrombectomy was associated with higher rates of independence in activities of daily living (mRS 0-2) than best medical therapy alone (122 [45.9%] of 266 vs 46 [19.3%] of 238; p < 0.0001). No significant difference between intervention and control groups was found when analysing either 90-day mortality (44 [16.5%] of 266 vs 46 [19.3%] of 238) or symptomatic intracerebral haemorrhage (14 [5.3%] of 266 vs eight [3.3%] of 239). No heterogeneity of treatment effect was noted across subgroups defined by age, gender, baseline stroke severity, vessel occlusion site, baseline Alberta Stroke Program Early CT Score, and mode of presentation; treatment effect was stronger in patients randomly assigned within 12-24 h (common OR 5.86 [95% CI 3.14-10.94]) than those randomly assigned within 6-12 h (1.76 [1.18-2.62]; pinteraction = 0.0087).
Interpretation
These findings strengthen the evidence for benefit of endovascular thrombectomy in patients with evidence of reversible cerebral ischaemia across the 6-24 h time window and are relevant to clinical practice. Our findings suggest that in these patients, thrombectomy should not be withheld on the basis of mode of presentation or of the point in time of presentation within the 6-24 h time window.
(4). Rajalakshmi V. Ground glass opacities are not always COVID-19: a case of acute eosinophilic pneumonitis caused by daptomycin. Lancet. 2022;399(10321):270.
An 83-year-old man was admitted to our hospital reporting a 3-week history of cough and exertional dyspnoea. At the time of admission, the patient was 4 weeks into a 6-week course of daptomycin and rifampicin because he had an infected prosthetic knee joint; he also had a history of pituitary hypoplasia and had not been vaccinated against SARS-CoV-2.
On examination, he was afebrile, his vital signs were normal, and there was no sign of active infection in the prosthetic knee joint.
Laboratory investigations showed a long-standing lymphopenia of 0.7×109 cells per L (normal 1.5-4.0) and a raised C-reactive protein (CRP) concentration of 186.5 mg/L (normal 0-5). The patient’s eosinophil cell count was 0.3×109 per L (normal 0-0.4), haemoglobin concentration 97 g/L (normal 130-170), and platelet cell count was 322×109 per L (normal 150-400). RT-PCR of four consecutive nasopharyngeal swabs for SARS-CoV-2 were negative; routine sputum culture and sensitivity, tuberculosis work-up, respiratory viral screen, and HIV serology were all negative.
Chest imaging-both x-ray and CT-showed bilateral, predominantly peripheral pulmonary infiltrates (figure).
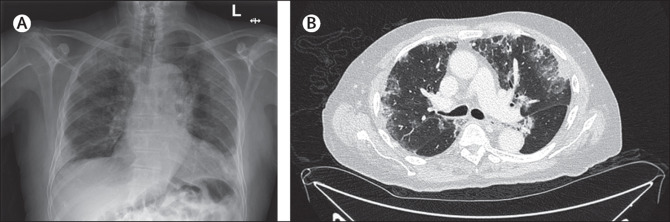
The patient was initially treated as presumed PCR-negative COVID-19 and the antibiotics were replaced with ceftriaxone; dexamethasone was not indicated because he was not hypoxic.
On day 7, due to persisting cough, a bronchoscopy was done; examination of the washings showed an eosinophilic infiltrate. A diagnosis of acute eosinophilic pneumonitis (AEP) was established through the modified Philit criteria: a respiratory illness of less than 1 month duration, pulmonary infiltrates on chest imaging, an eosinophilia of at least 25% in bronchoscopic washings, and the exclusion of other pulmonary eosinophilic diseases such as allergic bronchopulmonary aspergillosis and vasculitis. We thought parasitic infections were unlikely to be the cause-given the absence of a peripheral blood eosinophilia or any history of travel to a country where such infections are endemic. Additionally, the patient had no occupational or lifestyle exposure risk factors. And finally, x-ray and CT findings were not suggestive of fungal infections or allergic bronchopulmonary aspergillosis. Antineutrophil cytoplasmic antibody test was negative. The patient responded rapidly to high dose prednisolone and was allowed home 14 days after he was admitted. At follow-up 3 months later, the patient’s chest x-ray was normal.
Common causes of AEP include inhaled toxins-such as smoking, inhalational drugs, and dust-medications, and parasitic and fungal infections. Daptomycin therapy is a well recognised cause of eosinophilic pneumonitis, and we believe this was the most likely cause in our case.
Notably, the patient’s symptoms, biochemical, and radiological findings-including lymphopenia, raised CRP, and bilateral peripheral pulmonary infiltrates-pointed towards a diagnosis of PCR-negative COVID-19. Clinicians need to be on the look out for those subtle nuances that might indicate alternative diagnoses.
(5). Ware J, et al. Randomized Trial of Closed-Loop Control in Very Young Children with Type 1 Diabetes. N Engl J Med. 2022;386:209-19.
Background
The possible advantage of hybrid closed-loop therapy (i.e., artificial pancreas) over sensor-augmented pump therapy in very young children with type 1 diabetes is unclear.
Methods
In this multicenter, randomized, crossover trial, we recruited children 1 to 7 years of age with type 1 diabetes who were receiving insulin-pump therapy at seven centers across Austria, Germany, Luxembourg, and the United Kingdom. Participants received treatment in two 16-week periods, in random order, in which the closed-loop system was compared with sensor-augmented pump therapy (control). The primary end point was the between-treatment difference in the percentage of time that the sensor glucose measurement was in the target range (70 to 180 mg per deciliter) during each 16-week period. The analysis was conducted according to the intention-to-treat principle. Key secondary end points included the percentage of time spent in a hyperglycemic state (glucose level, >180 mg per deciliter), the glycated hemoglobin level, the mean sensor glucose level, and the percentage of time spent in a hypoglycemic state (glucose level, <70 mg per deciliter). Safety was assessed.
Results
A total of 74 participants underwent randomization. The mean (±SD) age of the participants was 5.6 ± 1.6 years, and the baseline glycated hemoglobin level was 7.3 ± 0.7%. The percentage of time with the glucose level in the target range was 8.7 percentage points (95% confidence interval [CI], 7.4 to 9.9) higher during the closed-loop period than during the control period (P < 0.001). The mean adjusted difference (closed-loop minus control) in the percentage of time spent in a hyperglycemic state was -8.5 percentage points (95% CI, -9.9 to -7.1), the difference in the glycated hemoglobin level was -0.4 percentage points (95% CI, -0.5 to -0.3), and the difference in the mean sensor glucose level was -12.3 mg per deciliter (95% CI, -14.8 to -9.8) (P < 0.001 for all comparisons). The time spent in a hypoglycemic state was similar with the two treatments (P = 0.74). The median time spent in the closed-loop mode was 95% (interquartile range, 92 to 97) over the 16-week closed-loop period. One serious adverse event of severe hypoglycemia occurred during the closed-loop period. One serious adverse event that was deemed to be unrelated to treatment occurred.
Conclusions
A hybrid closed-loop system significantly improved glycemic control in very young children with type 1 diabetes, without increasing the time spent in hypoglycemia.
(6). Feuerstadt P, et al. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N Engl J Med. 2022;386:220-229.
Background
Current therapies for recurrent Clostridioides difficile infection do not address the disrupted microbiome, which supports C. difficile spore germination into toxin-producing bacteria. SER-109 is an investigational microbiome therapeutic composed of purified Firmicutes spores for the treatment of recurrent C. difficile infection.
Methods
We conducted a phase 3, double-blind, randomized, placebo-controlled trial in which patients who had had three or more episodes of C. difficile infection (inclusive of the qualifying acute episode) received SER-109 or placebo (four capsules daily for 3 days) after standard-of-care antibiotic treatment. The primary efficacy objective was to show superiority of SER-109 as compared with placebo in reducing the risk of C. difficile infection recurrence up to 8 weeks after treatment. Diagnosis by toxin testing was performed at trial entry, and randomization was stratified according to age and antibiotic agent received. Analyses of safety, microbiome engraftment, and metabolites were also performed.
Results
Among the 281 patients screened, 182 were enrolled. The percentage of patients with recurrence of C. difficile infection was 12% in the SER-109 group and 40% in the placebo group (relative risk, 0.32; 95% confidence interval [CI], 0.18 to 0.58; P < 0.001 for a relative risk of < 1.0; P < 0.001 for a relative risk of < 0.833). SER-109 led to less frequent recurrence than placebo in analyses stratified according to age stratum (relative risk, 0.24 [95% CI, 0.07 to 0.78] for patients < 65 years of age and 0.36 [95% CI, 0.18 to 0.72] for those ≥ 65 years) and antibiotic received (relative risk, 0.41 [95% CI, 0.22 to 0.79] with vancomycin and 0.09 [95% CI, 0.01 to 0.63] with fidaxomicin). Most adverse events were mild to moderate and were gastrointestinal in nature, with similar numbers in the two groups. SER-109 dose species were detected as early as week 1 and were associated with bile-acid profiles that are known to inhibit C. difficile spore germination.
Conclusions
In patients with symptom resolution of C. difficile infection after treatment with standard-of-care antibiotics, oral administration of SER-109 was superior to placebo in reducing the risk of recurrent infection
(7). Finfer S, et al. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N Engl J Med. 2022
Background
Whether the use of balanced multielectrolyte solution (BMES) in preference to 0.9% sodium chloride solution (saline) in critically ill patients reduces the risk of acute kidney injury or death is uncertain.
Methods
In a double-blind, randomized, controlled trial, we assigned critically ill patients to receive BMES (Plasma-Lyte 148) or saline as fluid therapy in the intensive care unit (ICU) for 90 days. The primary outcome was death from any cause within 90 days after randomization. Secondary outcomes were receipt of new renal-replacement therapy and the maximum increase in the creatinine level during ICU stay.
Results
A total of 5037 patients were recruited from 53 ICUs in Australia and New Zealand – 2515 patients were assigned to the BMES group and 2522 to the saline group. Death within 90 days after randomization occurred in 530 of 2433 patients (21.8%) in the BMES group and in 530 of 2413 patients (22.0%) in the saline group, for a difference of -0.15 percentage points (95% confidence interval [CI], -3.60 to 3.30; P = 0.90). New renal-replacement therapy was initiated in 306 of 2403 patients (12.7%) in the BMES group and in 310 of 2394 patients (12.9%) in the saline group, for a difference of -0.20 percentage points (95% CI, -2.96 to 2.56). The mean (±SD) maximum increase in serum creatinine level was 36.6 ± 94.0 μmol per liter (0.41 ± 1.06 mg per deciliter) in the BMES group and 36.1 ± 90.2 μmol per liter (0.41 ± 1.02 mg per deciliter) in the saline group, for a difference of 0.5 μmol per liter (95% CI, -4.7 to 5.3) (0.01 mg per deciliter [95% CI, -0.05 to 0.06]). The number of adverse and serious adverse events did not differ meaningfully between the groups.
Conclusions
We found no evidence that the risk of death or acute kidney injury among critically ill adults in the ICU was lower with the use of BMES than with saline
(8). Beaton A, et al. Secondary Antibiotic Prophylaxis for Latent Rheumatic Heart Disease. N Engl J Med. 2022;386:230-40.
Background
Rheumatic heart disease affects more than 40.5 million people worldwide and results in 306,000 deaths annually. Echocardiographic screening detects rheumatic heart disease at an early, latent stage. Whether secondary antibiotic prophylaxis is effective in preventing progression of latent rheumatic heart disease is unknown.
Methods
We conducted a randomized, controlled trial of secondary antibiotic prophylaxis in Ugandan children and adolescents 5 to 17 years of age with latent rheumatic heart disease. Participants were randomly assigned to receive either injections of penicillin G benzathine (also known as benzathine benzylpenicillin) every 4 weeks for 2 years or no prophylaxis. All the participants underwent echocardiography at baseline and at 2 years after randomization. Changes from baseline were adjudicated by a panel whose members were unaware of the trial-group assignments. The primary outcome was echocardiographic progression of latent rheumatic heart disease at 2 years.
Results
Among 102,200 children and adolescents who had screening echocardiograms, 3327 were initially assessed as having latent rheumatic heart disease, and 926 of the 3327 subsequently received a definitive diagnosis on the basis of confirmatory echocardiography and were determined to be eligible for the trial. Consent or assent for participation was provided for 916 persons, and all underwent randomization; 818 participants were included in the modified intention-to-treat analysis, and 799 (97.7%) completed the trial. A total of 3 participants (0.8%) in the prophylaxis group had echocardiographic progression at 2 years, as compared with 33 (8.2%) in the control group (risk difference, -7.5 percentage points; 95% confidence interval, -10.2 to -4.7; P < 0.001). Two participants in the prophylaxis group had serious adverse events that were attributable to receipt of prophylaxis, including one episode of a mild anaphylactic reaction (representing <0.1% of all administered doses of prophylaxis).
Conclusions
Among children and adolescents 5 to 17 years of age with latent rheumatic heart disease, secondary antibiotic prophylaxis reduced the risk of disease progression at 2 years. Further research is needed before the implementation of population-level screening can be recommended.
(9). Ralph R. Managing snakebite. BMJ 2022;376.
What you need to know
Bites from venomous snakes can result in bleeding, paralysis, long term disability, and death.
Immobilise the bitten limb when transporting the patient to a medical facility; the universal use of pressure immobilisation is controversial, and tourniquets are not recommended.
The 20-minute whole blood clotting test is a simple bedside test to screen for and monitor coagulopathy in resource-limited settings.
Assess vital parameters and initiate resuscitation measures if the patient is clinically unstable with signs of bleeding, shock, paralysis, or respiratory distress.
Intravenous antivenom is recommended in patients with systemic symptoms; the dose and type depend on likely snake species, local guidelines, and availability.
(10). Tromp TR. Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study. 2022.
Background
Homozygous familial hypercholesterolaemia (HoFH) is a rare inherited disorder resulting in extremely elevated low-density lipoprotein cholesterol levels and premature atherosclerotic cardiovascular disease (ASCVD). Current guidance about its management and prognosis stems from small studies, mostly from high-income countries. The objective of this study was to assess the clinical and genetic characteristics, as well as the impact, of current practice on health outcomes of HoFH patients globally.
Methods
The HoFH International Clinical Collaborators registry collected data on patients with a clinical, or genetic, or both, diagnosis of HoFH using a retrospective cohort study design. This trial is registered with ClinicalTrials.gov, NCT04815005.
Findings
Overall, 751 patients from 38 countries were included, with 565 (75%) reporting biallelic pathogenic variants. The median age of diagnosis was 12.0 years (IQR 5.5-27.0) years. Of the 751 patients, 389 (52%) were female and 362 (48%) were male. Race was reported for 527 patients; 338 (64%) patients were White, 121 (23%) were Asian, and 68 (13%) were Black or mixed race. The major manifestations of ASCVD or aortic stenosis were already present in 65 (9%) of patients at diagnosis of HoFH. Globally, pretreatment LDL cholesterol levels were 14.7 mmol/L (IQR 11.6-18.4). Among patients with detailed therapeutic information, 491 (92%) of 534 received statins, 342 (64%) of 534 received ezetimibe, and 243 (39%) of 621 received lipoprotein apheresis. On-treatment LDL cholesterol levels were lower in high-income countries (3.93 mmol/L, IQR 2.6-5.8) versus non-high-income countries (9.3 mmol/L, 6.7-12.7), with greater use of three or more lipid-lowering therapies (LLT; high-income 66% vs non-high-income 24%) and consequently more patients attaining guideline-recommended LDL cholesterol goals (high-income 21% vs non-high-income 3%). A first major adverse cardiovascular event occurred a decade earlier in non-high-income countries, at a median age of 24.5 years (IQR 17.0-34.5) versus 37.0 years (29.0-49.0) in high-income countries (adjusted hazard ratio 1.64, 95% CI 1.13-2.38).
Interpretation
Worldwide, patients with HoFH are diagnosed too late, undertreated, and at high premature ASCVD risk. Greater use of multi-LLT regimens is associated with lower LDL cholesterol levels and better outcomes. Significant global disparities exist in treatment regimens, control of LDL cholesterol levels, and cardiovascular event-free survival, which demands a critical re-evaluation of global health policy to reduce inequalities and improve outcomes for all patients with HoFH.
