Journal scan: A review of 30 recent papers of immediate clinical significance, harvested from major international journals
From the desk of the Editor-in-Chief
(1). Robert F. Spiera, et al. Sarilumab for Relapse of Polymyalgia Rheumatica during Glucocorticoid Taper. N Engl J Med. 2023;389:1263-72.
Background
More than half of patients with polymyalgia rheumatica have a relapse during tapering of glucocorticoid therapy. Previous studies have suggested that interleukin-6 blockade may be clinically useful in the treatment of polymyalgia rheumatica. Sarilumab, a human monoclonal antibody, binds interleukin-6 receptor α and efficiently blocks the interleukin-6 pathway.
Methods
In this phase 3 trial, we randomly assigned patients in a 1:1 ratio to receive 52 weeks of a twice-monthly subcutaneous injection of either sarilumab (at a dose of 200 mg) plus a 14-week prednisone taper or placebo plus a 52-week prednisone taper. The primary outcome at 52 weeks was sustained remission, which was defined as the resolution of signs and symptoms of polymyalgia rheumatica by week 12 and sustained normalization of the C-reactive protein level, absence of disease flare, and adherence to the prednisone taper from weeks 12 through 52.
Results
A total of 118 patients underwent randomization (60 to receive sarilumab and 58 to receive placebo). At week 52, sustained remission occurred in 28% (17 of 60 patients) in the sarilumab group and in 10% (6 of 58 patients) in the placebo group (difference, 18 percentage points; 95% confidence interval, 4 to 32; P=0.02). The median cumulative glucocorticoid dose at 52 weeks was significantly lower in the sarilumab group than in the placebo group (777 mg vs. 2044 mg; P-0.001). The most common adverse events with sarilumab as compared with placebo were neutropenia (15% vs. 0%), arthralgia (15% vs. 5%), and diarrhea (12% vs. 2%). More treatment-related discontinuations were observed in the sarilumab group than in the placebo group (12% vs. 7%).
Conclusions
Sarilumab showed significant efficacy in achieving sustained remission and reducing the cumulative glucocorticoid dose in patients with a relapse of polymyalgia rheumatica during glucocorticoid tapering. (Funded by Sanofi and Regeneron Pharmaceuticals)
(2). Global Cardiovascular Risk Consortium, et al. Global Effect of Modifiable Risk Factors on Cardiovascular Disease and Mortality. N Engl J Med. 2023;389:1273-85.
Background
Five modifiable risk factors are associated with cardiovascular disease and death from any cause. Studies using individual-level data to evaluate the regional and sex-specific prevalence of the risk factors and their effect on these outcomes are lacking.
Methods
We pooled and harmonized individual-level data from 112 cohort studies conducted in 34 countries and 8 geographic regions participating in the Global Cardiovascular Risk Consortium. We examined associations between the risk factors (body-mass index, systolic blood pressure, non-high-density lipoprotein cholesterol, current smoking, and diabetes) and incident cardiovascular disease and death from any cause using Cox regression analyses, stratified according to geographic region, age, and sex. Population-attributable fractions were estimated for the 10-year incidence of cardiovascular disease and 10-year all-cause mortality.
Results
Among 1,518,028 participants (54.1% of whom were women) with a median age of 54.4 years, regional variations in the prevalence of the five modifiable risk factors were noted. Incident cardiovascular disease occurred in 80,596 participants during a median follow-up of 7.3 years (maximum, 47.3), and 177,369 participants died during a median follow-up of 8.7 years (maximum, 47.6). For all five risk factors combined, the aggregate global population-attributable fraction of the 10-year incidence of cardiovascular disease was 57.2% (95% confidence interval [CI], 52.4 to 62.1) among women and 52.6% (95% CI, 49.0 to 56.1) among men, and the corresponding values for 10-year all-cause mortality were 22.2% (95% CI, 16.8 to 27.5) and 19.1% (95% CI, 14.6 to 23.6).
Conclusions
Harmonized individual-level data from a global cohort showed that 57.2% and 52.6% of cases of incident cardiovascular disease among women and men, respectively, and 22.2% and 19.1% of deaths from any cause among women and men, respectively, may be attributable to five modifiable risk factors. (Funded by the German Center for Cardiovascular Research (DZHK)}
(3). Holger Thiele et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N Engl J Med. 2023;389:1286-97.
Background
Extracorporeal life support (ECLS) is increasingly used in the treatment of infarct-related cardiogenic shock despite a lack of evidence regarding its effect on mortality.
Methods
In this multicenter trial, patients with acute myocardial infarction complicated by cardiogenic shock for whom early revascularization was planned were randomly assigned to receive early ECLS plus usual medical treatment (ECLS group) or usual medical treatment alone (control group). The primary outcome was death from any cause at 30 days. Safety outcomes included bleeding, stroke, and peripheral vascular complications warranting interventional or surgical therapy.
Results
A total of 420 patients underwent randomization, and 417 patients were included in final analyses. At 30 days, death from any cause had occurred in 100 of 209 patients (47.8%) in the ECLS group and in 102 of 208 patients (49.0%) in the control group (relative risk, 0.98; 95% confidence interval [CI], 0.80 to 1.19; P=0.81). The median duration of mechanical ventilation was 7 days (interquartile range, 4 to 12) in the ECLS group and 5 days (interquartile range, 3 to 9) in the control group (median difference, 1 day; 95% CI, 0 to 2). The safety outcome consisting of moderate or severe bleeding occurred in 23.4% of the patients in the ECLS group and in 9.6% of those in the control group (relative risk, 2.44; 95% CI, 1.50 to 3.95); peripheral vascular complications warranting intervention occurred in 11.0% and 3.8%, respectively (relative risk, 2.86; 95% CI, 1.31 to 6.25).
Conclusions
In patients with acute myocardial infarction complicated by cardiogenic shock with planned early revascularization, the risk of death from any cause at the 30-day follow-up was not lower among the patients who received ECLS therapy than among those who received medical therapy alone. (Funded by the Else Kröner Fresenius Foundation)
(4). Hugo Francisco de Souza et al. Could infants’ robust immune responses reshape future vaccination strategies? https://doi.org/10.1016/j.cell.2023.08.044
Background
In a recent study published in the journal Cell, researchers employed a range of next-generation sequencing techniques, including the assay for transposase-accessible chromatin with sequencing (ATAC-seq) and single-cell RNA sequencing (scRNA-seq) to elucidate the system-wide immune responses of children in their first few weeks of life to infections. Their findings reveal that the immune responses of this unique cohort of individuals starkly contrast those of adults and, surprisingly, even those of young children aged five years and above.
While the former groups show a combination of both innate and acquired immune activation, infants present a solely innate immune response. These findings could help inform future child vaccination research and equip pediatricians with the knowledge to effectively manage infection in newborns.
Immunity and childhood infection
Immune responses are of two broad types – innate and acquired. The innate immune system is the body’s first line of defense against pathogens. This genetics-derived immunity responds in the same way to all germs and foreign substances, which is why it is sometimes referred to as the nonspecific immune system. In contrast, acquired immunity is also called specific immunity because it tailors its response to a specific antigen previously encountered. Its hallmarks are its ability to learn, adapt, and remember.
While innate immunity remains largely unchanged from birth through life, acquired immune response varies from individual to individual given specific antigen exposure, either through direct environmental exposure or vaccination. Antigen exposure, in turn, results in the priming of the immune system via antibody production. The type and abundance of these antibodies contribute to an adult’s ability to fight subsequent exposure to the same or highly similar antigens.
Prior research has revealed that immunity in adults and children performs significantly differently in both composition and function. The first few weeks of life are known to have a profound maturation effect on the subsequent immunity of a child through adulthood. While scientists have explored this maturation in children aged five and above, and recent work has attempted the same in healthy infants, no studies thus far have investigated the direct responses of newborns’ innate immunity to infections within the critical maturation period.
This distinction is essential to investigate given that children aged five have already developed a relatively mature immune system and thus may potentially respond very differently to infants without any immune system maturity.
About the study
In the present study, researchers used severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) data from infants to answer three primary questions – (1.) Since newborns only have nascent immune systems available to counter environmental infection, how do their T and B cells respond to and develop memory in the face of a pathogen? (2.) Pediatric coronavirus disease 2019 (COVID-19) has been observed to be much more symptomatically mild compared to adults’ disease response. Given this observation, what are the hallmarks of infants’ immune response that bring about these patterns?; (3.) Studies in adults and children have elucidated long-lasting epigenetic changes and autoimmune antibody generation. Are these patterns replicated in newborns?
To answer these questions, researchers employed a next-generation multi-omics approach to analyze and profile SARS-CoV-2 and its resultant immune activation in infants in their first few weeks and months of life. Blood (125 samples) from both infants and young children enrolled in the IMPRINT study cohort from the Cincinnati Children’s Hospital Medical Center were collected and screened weekly for COVID-19. These samples revealed 54 infection cases (case-cohort) and 27 uninfected controls. Blood analyses revealed that 32 infants were infected with pre-Omicron COVID-19 variants, while the remaining presented Omicron (or its variants).
Drug Discovery eBook NEW EDITION NOW OUT – Compilation of the top interviews, articles, and news in the last year.
To compare infant findings against those of adults, 62 blood samples representing 48 adult COVID-19 cases and 10 healthy controls were collected from the Hope Clinic at Emory University in Atlanta, and 47 blood samples representing 41 infected mothers and 3 healthy controls from the Stanford University Medical Centre.
Anti-Spike electrochemiluminescence (ECL) binding enzyme-linked immunosorbent assay (ELISA) was used to investigate antibody titers (both binding and neutralizing) against pre- and Omicron COVID-19 variants. Autoantibody response was subsequently measured using a custom ELISA against IFNa2 analysis of plasma samples. To further elucidate the adaptive immune responses in infants, the kinetics of COVID-19-specific memory T and B cells were investigated.
Since severe COVID-19 infection has been previously shown to follow dysregulations in innate immune responses (in adults), researchers measured the kinetics of plasma cytokine responses using principal-component analysis (PCA) of cytokines separated by type and abundance. To elucidate the cellular dynamics of immune responses, mass cytometry was employed to analyze peripheral blood leukocytes. This analysis was followed up by single-cell RNA sequencing (scRNA-seq) to further insights into the activation state of various immune cells. Herein, gene expression and chromatin accessibility profiles of adults before, during, and after were compared with those of infants displaying mild infection.
The assay for transposase-accessible chromatin with sequencing (ATAC-seq) was used to reveal system-wide modifications in chromatic accessibility during COVID-19. Finally, nasal swab samples were analyzed through PCA to determine the differences between newborns’ and young children’s mucosal immune responses.
Study findings
This multi-omics study revealed that while antibodies against COVID-19 rapidly decay in adults (allowing for repeat infection from the same COVID-19 variant), memory T and B cells in infants depict robust and long-lasting responses against SARS-CoV-2. While adult antibodies are known to almost completely decay in 120 days following COVID-19 infection, infants’ antibodies showed almost no change in ELISA titers over the 300-day duration of this study. This data is confounded by the differences in results from pre-Omicron and Omicron SARS-CoV-2 variants of concern (VOCs).
While antibodies against the former did not decay over time, B cells against the latter did. Additionally, T cells, known to mutate and evolve at a slow rate, did not change on infection by an Omicron variant following pre-Omicron infection, suggesting that while infants’ innate immunity far outperforms that of adults in the COVID-19 context (none of the infants under study ever presented severe COVID-19 symptoms), they immune systems may potentially be overwhelmed on repeated COVID-19 exposure comprising different VOCs.
This study revealed three key findings regarding infant innate immune response – (1.) Infants and young children (median age 5 years) depict very different mucosal and systemic immune responses. While children and adults present high levels of TNF-a, (interleukin) IL-6, OSM, EN-RAGE, and other inflammatory mediators in the nasal mucosa, infants depict high levels of type I and II interferons (IFNs), inflammatory cytokines (IL-6, IL-8, TNF-a, and IL-17C), and various chemokines.
(2.) Infants and children presented musical immune responses characterized by chemokines and cytokines associated with the Th17 response type. When combined with high observed neutrophil densities in processed blood samples, these findings suggest crosstalk between Th 17 cells and neutrophils, which may play a crucial role in both the innate and adaptive immunity of infants against subsequent COVID-19 infection. (3.) Innate immunity in infants was rapidly activated in both mucosal and systemic systems, contrasting patterns of slow recruitment and defective plasmacytoid dendritic- and myeloid cells during initial SARS-CoV-2 exposure.
Taken together, these findings suggest that the rapid induction of mucosal immunity in the nasal tract might contribute to the mild course of disease in infants and young children by containing viral replication in the nose.
Conclusions
In the present study, the research employed a multi-omics approach to investigate infant immune response to COVID-19 infection for the first time. Results highlight a stark contrast between the immune responses of infants and adults, both innate (wherein infants’ immune systems are recruited far more rapidly and effectively than those of adults) and acquired (wherein memory cell decay in infants is much slower than that observed in adults). This implies that against highly virulent pathogens, including COVID-19, the immune systems of infants during their first year of life far outperform that of adults and, surprisingly, even the more mature immune systems of children five years their senior.
This raises the prospect of devising vaccine adjuvants that target such non-canonical pathways of innate activation to stimulate persistent antibody responses, without the collateral immunopathology that often results from unwanted inflammation.
(5). Joseph Donovan, et al. Adjunctive Dexamethasone for Tuberculous Meningitis in HIV-Positive Adults. N Engl J Med. 2023;389:1357-67.
Background
Adjunctive glucocorticoids are widely used to treat human immunodeficiency virus (HIV)-associated tuberculous meningitis despite limited data supporting their safety and efficacy.
Methods
We conducted a double-blind, randomized, placebo-controlled trial involving HIV-positive adults (18 years of age) with tuberculous meningitis in Vietnam and Indonesia. Participants were randomly assigned to receive a 6-to-8-week tapering course of either dexamethasone or placebo in addition to 12 months of antituberculosis chemotherapy. The primary end point was death from any cause during the 12 months after randomization.
Results
A total of 520 adults were randomly assigned to receive either dexamethasone (263 participants) or placebo (257 participants). The median age was 36 years; 255 of 520 participants (49.0%) had never received antiretroviral therapy, and 251 of 484 participants (51.9%) with available data had a baseline CD4 count of 50 cells per cubic millimeter or less. Six participants withdrew from the trial, and five were lost to follow-up. During the 12 months of follow-up, death occurred in 116 of 263 participants (44.1%) in the dexamethasone group and in 126 of 257 participants (49.0%) in the placebo group (hazard ratio, 0.85; 95% confidence interval, 0.66 to 1.10; P=0.22). Prespecified analyses did not reveal a subgroup that clearly benefited from dexamethasone. The incidence of secondary end-point events, including cases of immune reconstitution inflammatory syndrome during the first 6 months, was similar in the two trial groups. The numbers of participants with at least one serious adverse event were similar in the dexamethasone group (192 of 263 participants [73.0%]) and the placebo group (194 of 257 participants [75.5%]) (P=0.52).
Conclusions
Among HIV-positive adults with tuberculous meningitis, adjunctive dexamethasone, as compared with placebo, did not confer a benefit with respect to survival or any secondary endpoint. (Funded by the Wellcome Trust)
(6). Tara T.M. Lee, et al. Automated Insulin Delivery in Women with Pregnancy Complicated by Type 1 Diabetes. N Engl J Med. 2023;389(17):1566-78.
Background
Hybrid closed-loop insulin therapy has shown promise for the management of type 1 diabetes during pregnancy; however, its efficacy is unclear.
Methods
In this multicenter, controlled trial, we randomly assigned pregnant women with type 1 diabetes and a glycated hemoglobin level of at least 6.5% at nine sites in the United Kingdom to receive standard insulin therapy or hybrid closed-loop therapy, with both groups using continuous glucose monitoring. The primary outcome was the percentage of time in the pregnancy-specific target glucose range (63 to 140 mg per deciliter [3.5 to 7.8 mmol per liter]) as measured by continuous glucose monitoring from 16 weeks’ gestation until delivery. Analyses were performed according to the intention-to-treat principle. Key secondary outcomes were the percentage of time spent in a hyperglycemic state (glucose level >140 mg per deciliter), overnight time in the target range, the glycated hemoglobin level, and safety events.
Results
A total of 124 participants with a mean (+SD) age of 31.1+5.3 years and a mean baseline glycated hemoglobin level of 7.7+1.2% underwent randomization. The mean percentage of time that the maternal glucose level was in the target range was 68.2+10.5% in the closed-loop group and 55.6+12.5% in the standard-care group (mean adjusted difference, 10.5 percentage points; 95% confidence interval [CI], 7.0 to 14.0; P-0.001). Results for the secondary outcomes were consistent with those of the primary outcome; participants in the closed-loop group spent less time in a hyperglycemic state than those in the standard-care group (difference, -10.2 percentage points; 95% CI, -13.8 to -6.6); had more overnight time in the target range (difference, 12.3 percentage points; 95% CI, 8.3 to 16.2), and had lower glycated hemoglobin levels (difference, -0.31 percentage points; 95% CI, -0.50 to -0.12). Little time was spent in a hypoglycemic state. No unanticipated safety problems associated with the use of closed-loop therapy during pregnancy occurred (6 instances of severe hypoglycemia, vs. 5 in the standard-care group; 1 instance of diabetic ketoacidosis in each group; and 12 device-related adverse events in the closed-loop group, 7 related to closed-loop therapy).
Conclusions
Hybrid closed-loop therapy significantly improved maternal glycemic control during pregnancy complicated by type 1 diabetes. (Funded by the Efficacy and Mechanism Evaluation Program)
(7). Loren G. Miller, et al. Decolonization in Nursing Homes to Prevent Infection and Hospitalization. N Engl J Med 2023;389:1766-77
Background
Nursing home residents are at high risk for infection, hospitalization, and colonization with multidrug-resistant organisms.
Methods
We performed a cluster-randomized trial of universal decolonization as compared with routine-care bathing in nursing homes. The trial included an 18-month baseline period and an 18-month intervention period. Decolonization entailed the use of chlorhexidine for all routine bathing and showering and administration of nasal povidone-iodine twice daily for the first 5 days after admission and then twice daily for 5 days every other week. The primary outcome was transfer to a hospital due to infection. The secondary outcome was transfer to a hospital for any reason. An intention-to-treat (as-assigned) difference-in-differences analysis was performed for each outcome with the use of generalized linear mixed models to compare the intervention period with the baseline period across trial groups.
Results
Data were obtained from 28 nursing homes with a total of 28,956 residents. Among the transfers to a hospital in the routine-care group, 62.2% (the mean across facilities) were due to infection during the baseline period and 62.6% were due to infection during the intervention period (risk ratio, 1.00; 95% confidence interval [CI], 0.96 to 1.04). The corresponding values in the decolonization group were 62.9% and 52.2% (risk ratio, 0.83; 95% CI, 0.79 to 0.88), for a difference in risk ratio, as compared with routine care, of 16.6% (95% CI, 11.0 to 21.8; P-0.001). Among the discharges from the nursing home in the routine-care group, transfer to a hospital for any reason accounted for 36.6% during the baseline period and for 39.2% during the intervention period (risk ratio, 1.08; 95% CI, 1.04 to 1.12). The corresponding values in the decolonization group were 35.5% and 32.4% (risk ratio, 0.92; 95% CI, 0.88 to 0.96), for a difference in risk ratio, as compared with routine care, of 14.6% (95% CI, 9.7 to 19.2). The number needed to treat was 9.7 to prevent one infection-related hospitalization and 8.9 to prevent one hospitalization for any reason.
Conclusions
In nursing homes, universal decolonization with chlorhexidine and nasal iodophor led to a significantly lower risk of transfer to a hospital due to infection than routine care. (Funded by the Agency for Healthcare Research and Quality
(8). Tamar Schiff et al. Next Steps for Clinical Xenotransplantation. https://doi.org/10.7326/M23-1823
Clinical xenotransplantation transplantation of tissues from nonhuman animals into humans offers the potential to mitigate the morbidity and mortality caused by the dire shortage of human organs. Pigs are the primary xenograft source of interest because they are easy to breed and the size and function of their organs are appropriate for use in humans. Through gene editing, porcine organs have been altered to overcome major human immunologic barriers, with research focused on initial clinical application of kidneys and hearts. As interest in clinical translation of the science grows, substantial concern remains about the risk for xenozoonosis infection of xenograft recipients with microorganisms
(9). Prof Martin J van den Bent et al. Primary brain tumours in adults. The Lancet. 2023;402(10412):1564-79.
The most frequent adult-type primary CNS tumours are diffuse gliomas, but a large variety of rarer CNS tumour types exists. The classification of these tumours is increasingly based on molecular diagnostics, which is reflected in the extensive molecular foundation of the recent WHO 2021 classification of CNS tumours. Resection as extensive as is safely possible is the cornerstone of treatment in most gliomas, and is now also recommended early in the treatment of patients with radiological evidence of histologically low-grade tumours. For the adult-type diffuse glioma, standard of care is a combination of radiotherapy and chemotherapy. Although treatment with curative intent is not available, combined modality treatment has resulted in long-term survival (>10-20 years) for some patients with isocitrate dehydrogenase (IDH) mutant tumours. Other rarer tumours require tailored approaches, best delivered in specialised centres. Targeted treatments based on molecular alterations still only play a minor role in the treatment landscape of adult-type diffuse glioma, and today are mainly limited to patients with tumours with BRAFV600E (ie, Val600Glu) mutations. Immunotherapy for CNS tumours is still in its infancy, and so far, trials with checkpoint inhibitors and vaccination studies have not shown improvement in patient outcomes in glioblastoma. Current research is focused on improving our understanding of the immunosuppressive tumour environment, the molecular heterogeneity of tumours, and the role of tumour microtube network connections between cells in the tumour microenvironment. These factors all appear to play a role in treatment resistance, and indicate that novel approaches are needed to further improve outcomes of patients with CNS tumours.
(10). Prof Raj R Makkar et al. Outcomes of repeat transcatheter aortic valve replacement with balloon-expandable valves: a registry study. The Lancet. 2023; 402(10412): 1529-40.
Background
With increasing numbers of patients undergoing transcatheter aortic valve replacement (TAVR), data on management of failed TAVR, including repeat TAVR procedure, are needed. The aim of this study was to assess the safety and efficacy of redo-TAVR in a national registry.
Methods
This study included all consecutive patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry from Nov 9, 2011, to Dec 30, 2022 who underwent TAVR with balloon-expandable valves in failed transcatheter heart valves (redo-TAVR) or native aortic valves (native-TAVR). Procedural, echocardiographic, and clinical outcomes were compared between redo-TAVR and native-TAVR cohorts using propensity score matching.
Findings
Among 350 591 patients (1320 redo-TAVR; 349 271 native-TAVR), 1320 propensity-matched pairs of patients undergoing redo-TAVR and native-TAVR were analysed (redo-TAVR cohort: mean age 78 years [SD 9]; 559 [42.3%] of 1320 female, 761 [57.7%] male; mean predicted surgical risk of 30-day mortality 8.1%). The rates of procedural complications of redo-TAVR were low (coronary compression or obstruction: four [0.3%] of 1320; intraprocedural death: eight [0.6%] of 1320; conversion to open heart surgery: six [0.5%] of 1319) and similar to native-TAVR. There was no significant difference between redo-TAVR and native-TAVR populations in death at 30 days (4.7% vs 4.0%, p=0.36) or 1 year (17.5% vs 19.0%, p=0.57), and stroke at 30 days (2.0% vs 1.9%, p=0.84) or 1 year (3.2% vs 3.5%, p=0.80). Redo-TAVR reduced aortic valve gradients at 1 year, although they were higher in the redo-TAVR group compared with the native-TAVR group (15 mm Hg vs 12 mm Hg; p-0.0001). Moderate or severe aortic regurgitation rates were similar between redo-TAVR and native-TAVR groups at 1 year (1.8% vs 3.3%, p=0.18). Death or stroke after redo-TAVR were not significantly affected by the timing of redo-TAVR (before or after 1 year of index TAVR), or by index transcatheter valve type (balloon-expandable or non-balloon-expandable).
Interpretation
Redo-TAVR with balloon-expandable valves effectively treated dysfunction of the index TAVR procedure with low procedural complication rates, and death and stroke rates similar to those in patients with a similar clinical profile and predicted risk undergoing TAVR for native aortic valve stenosis. Redo-TAVR with balloon-expandable valves might be a reasonable treatment for failed TAVR in selected patients.
(11). Huimin Chen et al. Thalidomide for Recurrent Bleeding Due to Small-Intestinal Angiodysplasia. N Engl J Med. 2023;389:1649-59.
Background
Recurrent bleeding from the small intestine accounts for 5 to 10% of cases of gastrointestinal bleeding and remains a therapeutic challenge. Thalidomide has been evaluated for the treatment of recurrent bleeding due to small-intestinal angiodysplasia (SIA), but confirmatory trials are lacking.
Methods
We conducted a multicenter, double-blind, randomized, placebo-controlled trial to investigate the efficacy and safety of thalidomide for the treatment of recurrent bleeding due to SIA. Eligible patients with recurrent bleeding (at least four episodes of bleeding during the previous year) due to SIA were randomly assigned to receive thalidomide at an oral daily dose of 100 mg or 50 mg or placebo for 4 months. Patients were followed for at least 1 year after the end of the 4-month treatment period. The primary end point was effective response, which was defined as a reduction of at least 50% in the number of bleeding episodes that occurred during the year after the end of thalidomide treatment as compared with the number that occurred during the year before treatment. Key secondary end points were cessation of bleeding without rebleeding, blood transfusion, hospitalization because of bleeding, duration of bleeding, and hemoglobin levels.
Results
Overall, 150 patients underwent randomization: 51 to the 100-mg thalidomide group, 49 to the 50-mg thalidomide group, and 50 to the placebo group. The percentages of patients with an effective response in the 100-mg thalidomide group, 50-mg thalidomide group, and placebo group were 68.6%, 51.0%, and 16.0%, respectively (P-0.001 for simultaneous comparison across the three groups). The results of the analyses of the secondary end points supported those of the primary end point. Adverse events were more common in the thalidomide groups than in the placebo group overall; specific events included constipation, somnolence, limb numbness, peripheral edema, dizziness, and elevated liver-enzyme levels.
Conclusions
In this placebo-controlled trial, treatment with thalidomide resulted in a reduction in bleeding in patients with recurrent bleeding due to SIA. (Funded by the National Natural Science Foundation of China
(12). Hagit Baris Feldman et al. CORIN and Left Atrial Cardiomyopathy, Hypertension, Arrhythmia, and Fibrosis. N Engl J Med. 2023;389:1685-92.
Two siblings presented with cardiomyopathy, hypertension, arrhythmia, and fibrosis of the left atrium. Each had a homozygous null variant in CORIN, the gene encoding atrial natriuretic peptide (ANP)-converting enzyme. A plasma sample obtained from one of the siblings had no detectable levels of corin or N-terminal pro-ANP but had elevated levels of B-type natriuretic peptide (BNP) and one of the two protein markers of fibrosis that we tested. These and other findings support the hypothesis that BNP cannot fully compensate for a lack of activation of the ANP pathway and that corin is critical to normal ANP activity, left atrial function, and cardiovascular homeostasis.
(13). Amy S Shah et al. Weekly insulin: a paradigm shift in type 1 diabetes therapy. The Lancet. 2023;402(10413):1598-99.
Basal insulin treatment is the cornerstone of type 1 diabetes management and often becomes necessary over time in type 2 diabetes as the disease progresses.1 Basal insulins are administered once or twice daily; however, there is potential added value from a once-weekly insulin to reduce treatment burden.2 Insulin icodec (icodec) is one of two once-weekly insulins in late phase clinical development. Icodec is an insulin analogue with three substitutions to the amino acid structure and an attached C20 icosane fatty diacid chain that allows the molecule to bind reversibly to albumin, prolonging the half-life to 196 h (approximately 7 days) and achieving steady state after 3-4 once-weekly injections.3
The ONWARDS clinical trial programme is a series of phase 3a trials designed to evaluate the efficacy and safety of once-weekly icodec compared with available daily basal insulin analogues in adults with type 1 and type 2 diabetes across various therapeutic settings.4 ONWARDS 1-5 studied adults with type 2 diabetes; ONWARDS 6 is believed to be the first and only phase 3a trial to report outcomes following treatment of once-weekly basal insulin in adults with type 1 diabetes. In The Lancet, David Russell-Jones and colleagues report the results of ONWARDS 6, a multinational, randomised, open-label, treat-to-target, 26-week trial (plus 26 weeks of extension), which evaluated once-weekly icodec versus once-daily insulin degludec (degludec), both in combination with mealtime insulin aspart in 582 people (337 men [58%], 245 women [42%]; 123 Asian, 11 Black or African American, and 448 White) with type 1 diabetes. Both icodec and degludec doses were adjusted throughout the trial to a prespecified titration algorithm to attain a pre-breakfast self-monitored blood glucose target of 4.4-7.2 mmol/l (80-130 mg/dL). Icodec achieved its primary endpoint (HbA1c reduction) showing non-inferiority in reducing HbA1c at week 26 compared with degludec (0.47 percentage points vs 0.51 percentage points, respectively; estimated treatment difference [ETD] 0.05 percentage points [95% CI -0.13 to 0.23]). At week 52, estimated mean HbA1c reduction was lower with icodec than degludec (icodec -0.37 percentage points vs degludec, -0.54 percentage points; ETD 0.17 percentage points [95% CI 0.02 to 0.31]; p=0.021). During the trial and extension, rates of combined clinically significant (blood glucose -3.0 mmol/L [54 mg/dL]) and severe hypoglycaemia (requiring external assistance for recovery) were higher with icodec compared with degludec (estimated rate ratio 1.80 [95% CI 1.48 to 2.18]; p-0.0001).
Several aspects related to hypoglycaemia in this trial warrant further elaboration. Although rates of hypoglycaemia were higher with icodec versus degludec, episodes of severe hypoglycaemia were low overall and observed in 4% of participants receiving icodec and 4% receiving degludec. Hypoglycaemia event rates were lower than previously published treat-to-target studies investigating degludec in patients with type 1 diabetes.5 Furthermore, hypoglycaemia with icodec might be related to the intensive treat-to-target algorithm utilised in this trial. Additionally, although participants wore continuous glucose monitor for the duration of the trial, insulin doses were titrated on the basis of pre-breakfast blood glucose measurements; optimal titration based on continuous glucose monitors data might reduce hypoglycaemia. Further, slower weekly titration of icodec in the phase 2 icodec studies reduced the risk for hypoglycaemia while maintaining adequate glycaemic control, suggesting future ways to reduce hypoglycaemia with icodec.6
Once-weekly insulin offers potential advantages, but who will benefit most? This is more evident for patients with type 2 diabetes. Once-weekly insulin injections are consistent with the growing popularity of once-weekly GLP-1 receptor agonists with the potential to combine the two for weight and cardiovascular benefits. In the initial ONWARDS trials of over 4200 participants with type 2 diabetes, the efficacy and safety of icodec was similar to once-daily basal insulin analogues and HbA1c reduction was superior for icodec among insulin-naive participants (ONWARDS 1, 3, and 5) and in those switching from a daily basal insulin (ONWARDS 2).4 For patients with type 1 diabetes, the risk of hypoglycaemia and less-frequent, weekly dose titration might render it a less attractive option, especially in comparison with methods that use automated insulin delivery systems that fine-tune glucose concentrations and are designed to reduce hypoglycaemia.7, 8 But there are people with type 1 diabetes who might benefit. For example, for patients who struggle with adherence, this might be an opportunity to improve glycaemic control. Additionally, having a relatively constant concentration of basal insulin might reduce the frequency of diabetic ketoacidosis, and prevent related hospitalisations. Finally, multiple daily insulin injections (with basal insulin) remain the most commonly used treatment worldwide,9, 10 so fewer injections for people receiving multiple-dose injection therapy are inherently desirable and might reduce the burden of daily diabetes management.
Future work is needed to learn how to optimally initiate and titrate once-weekly insulin, manage concomitant preprandial insulin, and adjust once-weekly insulin in the face of exercise frequency and intensity, sick days, and surgery. Although important clinical questions remain, reducing the number of basal insulin injections from 365 to 52 administrations per year remains a substantial innovation in insulin management.
(14). Syed Mahamad et al. Immune thrombocytopenia, The Harrington Experiment. https://doi.org/10.1016/S0140-6736(23)01836-6.
Perhaps the most convincing study on the mechanism of immune thrombocytopenia was the classic Harrington experiment in 1950. William Harrington collected a cohort of healthy volunteers and transfused into each of them plasma or whole blood from people with immune thrombocytopenia. Most participants (including Harrington himself) developed severe, rapid onset, and transient thrombocytopenia. The experiment spurred decades of studies in search of the plasma factor that causes immune thrombocytopenia.
(15). Emma Doble et al. Patient partnership at BMJ. BMJ. 2023; 383:2505.
The experience of patients and the public can and should contribute to finding solutions to the pressure on health services. But their voices are often marginalised.
Solid foundation
The BMJ has been a vocal champion of patient partnership for over 20 years.
In 2014 our focus shifted to developing in-house expertise, policies, and capabilities, including patient editors, a patient advisory panel, peer review by patients, co-production of articles, and firmer requirements for authors of research studies to include a statement on patient and public involvement.
By 2022, more than 85% of research papers were sent for patient and public review, and around 50% of accepted research had a completed patient and public review. Articles in the Education section have a similar rate of patient and public review. Researchers reporting no patient and public involvement must now detail the barriers they faced, to inform future progress.
We continue to give patients a prominent voice in the journal through our What your patient is thinking series6 and increasing the number of patient commentaries and opinion pieces. BMJ articles with a patient coauthor are free to access for two weeks, and patient author groups without access to funding can also apply for a waiver of BMJ’s open access fees. This year we have also started using a patient author or public representative affiliation for patient and public authors to help highlight, quantify, and track the patient authored content we publish.
Priority areas
In addition, our international patient and public advisory panel has been working to identify priority areas for advocacy. After the panel identified patient access to health records as a priority in 2021, we published a collection of articles and hosted a series of webinars exploring some of the remaining challenges. Despite progress in some countries, many people around the world are still unable to access their health records. We will continue to champion patients’ right to access their health records, share progress, and offer a platform for discussion.
We have now added four further priority areas to our work, starting with patient safety. As healthcare systems attempt to rebuild after the covid-19 pandemic, waiting lists lengthen, staff shortages deepen, and concerns for patient safety grow. Our aim is to reinstate the patient voice in healthcare decision making after it was sidelined during the pandemic and to highlight where this is being done well. Several recent high profile failures in patient safety, including maternity scandals where hospitals and units failed to listen to women and their families, show why this crucial.
The second priority area is patient involvement in genomic medicine. The far reaching effects of this rapidly evolving field on patients, families, and the public are not being adequately considered. Progress in the science is rapidly outpacing the development of strategies for safe and ethical implementation. The challenges are clear given the sensitive information that may be identified when using genomic testing and medicine.
The third priority is patient involvement in medical conferences. We will re-energise the patients included charter12 for conferences first published in 2015, as a way for organisers to demonstrate their commitment to patient and public involvement by reviewing the charter’s clauses and championing organisations that meet their requirements in full. We will also encourage more organisations to commit to including patients and the public in their conferences.
Finally, patient editors and panel members will support The BMJ’s wider work on sustainability by drawing attention to patient-led initiatives on sustainable healthcare. As always, we welcome readers’ thoughts, ideas, and comments on any of these topics.
The BMJ remains committed to patient and public partnership, and we plan to grow and diversify our database of patient contributors to ensure we publish content relevant to diverse international patients and carers. A patient-focused special issue is underway to mark the 10-year anniversary of our patient and public partnership strategy in 2024.
With the support of our international patient and public advisory panel, The BMJ will continue to be a leading voice on the science and implementation of working collaboratively with patients, families, carers, and the public. Working together is essential to achieve our mission to improve the health and well-being of people and the planet. Involving patients in the day-to-day work of a scientific journal is hard, complex, and requires commitment, but it is ultimately rewarding and essential.
(16). The world’s first vaccine for chikungunya, an emerging global health threat.
The mosquito-borne disease causes fever and joint pains and can be fatal to newborns.
This year, about 440,000 chikungunya cases, including 350 deaths, have been reported as of September.
There is currently no specific drug to treat chikungunya. South America and South Asia have seen the most number of cases this year.
The vaccine named Ixchiq has been approved by US FDA for those aged 18 and above and are at high risk of contracting the disease, the FDA said on Friday. It was developed by Europe’s Valneva and will be administered in a single shot.
Infection with chikungunya virus can lead to severe disease and prolonged health problems, particularly for older adults and individuals with underlying medical conditions
At least five million chikungunya cases have been reported since 2008. Other symptoms include rashes, headaches and muscle pain. Joint pains can persist for months or even years.
People in tropical and subtropical regions of Africa, Southeast Asia, and parts of the Americas are at the highest risk of infection because mosquitos carrying the chikungunya virus are endemic in these areas.
However, chikungunya virus has spread to new geographical areas causing a rise in global prevalence of the disease,
Data from the European Centre for Disease Prevention and Control showed that Brazil has had the highest number of cases so far this year with 218,613.
More than 93,000 cases have also been reported in India, where the capital Delhi saw a large outbreak in 2016.
(17). Adrian O’Dowd. Regular visits from friends and family protect against dying, finds a study. BMJ 2023; 383:2634.
A lack of visits from friends or family has been linked with an increased risk of dying in an analysis of more than 450,000 people involved in the UK Biobank study. Identifying those at a higher risk of dying because of social factors could allow for more targeted interventions, argues the researchers.
Previous research has identified associations between deaths from any cause and both a sense of loneliness and living alone, but the combined impacts of different types of social interaction on mortality have been unclear.
Researchers from the University of Glasgow used data from 458,146 adults recruited to the UK Biobank to investigate the association between mortality and five types of social interaction.
(18). Jiawen Dong et al. Polycystic ovary syndrome: pathophysiology and therapeutic opportunities. BMJ Med. 2023;2:e000548.
Polycystic ovary syndrome is characterised by excessive levels of androgens and ovulatory dysfunction, and is a common endocrine disorder in women of reproductive age. Polycystic ovary syndrome arises as a result of polygenic susceptibility in combination with environmental influences that might include epigenetic alterations and in utero programming. In addition to the well recognised clinical manifestations of hyperandrogenism and ovulatory dysfunction, women with polycystic ovary syndrome have an increased risk of adverse mental health outcomes, pregnancy complications, and cardiometabolic disease. Unlicensed treatments have limited efficacy, mostly because drug development has been hampered by an incomplete understanding of the underlying pathophysiological processes. Advances in genetics, metabolomics, and adipocyte biology have improved our understanding of key changes in neuroendocrine, enteroendocrine, and steroidogenic pathways, including increased gonadotrophin releasing hormone pulsatility, androgen excess, insulin resistance, and changes in the gut microbiome. Many patients with polycystic ovary syndrome have high levels of 11-oxygenated androgens, with high androgenic potency, that might mediate metabolic risk. These advances have prompted the development of new treatments, including those that target the neurokinin-kisspeptin axis upstream of gonadotrophin releasing hormone, with the potential to lessen adverse clinical sequelae and improve patient outcomes.
(19). Karine Lacombe et al. Use of COVID-19 convalescent plasma to treat patients admitted to hospital for COVID-19 with or without underlying immunodeficiency: open label, randomised clinical trial. http://orcid.org/0000-0001-8772-9029.
Objective To evaluate the efficacy of covid-19 convalescent plasma to treat patients admitted to hospital for moderate covid-19 disease with or without underlying immunodeficiency (CORIPLASM trial).
Design Open label, randomised clinical trial.
Setting CORIMUNO-19 cohort (publicly supported platform of open label, randomised controlled trials of immune modulatory drugs in patients admitted to hospital with moderate or severe covid-19 disease) based on 19 university and general hospitals across France, from 16 April 2020 to 21 April 2021.
Participants
120 adults (n=60 in the covid-19 convalescent plasma group, n=60 in the usual care group) admitted to hospital with a positive SARS-CoV2 test result, duration of symptoms -9,days, and World Health Organization score of 4 or 5. 49 patients (n=22, n=27) had underlying immunosuppression.
Interventions
Open label randomisation to usual care or four units (200-220 mL/unit, 2 units/day over two consecutive days) of covid-19 convalescent plasma with a seroneutralisation titre >40.
Main outcome measures Primary outcomes were proportion of patients with a WHO Clinical Progression Scale score of 6 on the 10 point scale on day 4 (higher values indicate a worse outcome), and survival without assisted ventilation or additional immunomodulatory treatment by day 14. Secondary outcomes were changes in WHO Clinical Progression Scale scores, overall survival, time to discharge, and time to end of dependence on oxygen supply. Predefined subgroups analyses included immunosuppression status, duration of symptoms before randomisation, and use of steroids.
Results
A 120 patients were recruited and assigned to covid-19 convalescent plasma (n=60) or usual care (n=60), including 22 (covid-19 convalescent plasma) and 27 (usual care) patients who were immunocompromised. 13 (22%) patients who received convalescent plasma had a WHO Clinical Progression Scale score of 6 at day 4 versus eight (13%) patients who received usual care (adjusted odds ratio 1.88, 95% credible interval 0.71 to 5.24). By day 14, 19 (31.6%) patients in the convalescent plasma group and 20 (33.3%) patients in the usual care group needed ventilation, additional immunomodulatory treatment, or had died. For cumulative incidence of death, three (5%) patients in the convalescent plasma group and eight (13%) in the usual care group died by day 14 (adjusted hazard ratio 0.40, 95% confidence interval 0.10 to 1.53), and seven (12%) patients in the convalescent plasma group and 12 (20%) in the usual care group by day 28 (adjusted hazard ratio 0.51, 0.20 to 1.32). In a subgroup analysis performed in patients who were immunocompromised, transfusion of covid-19 convalescent plasma was associated with mortality (hazard ratio 0.39, 95% confidence interval 0.14 to 1.10).
Conclusions
In this study, covid-19 convalescent plasma did not improve early outcomes in patients with moderate covid-19 disease. The efficacy of convalescent plasma in patients who are immunocompromised should be investigated further.
(20). Jane C.Y. Wong et al. Large-Bowel Obstruction from Hereditary Angioedema. N Engl J Med 2023;389:e4.
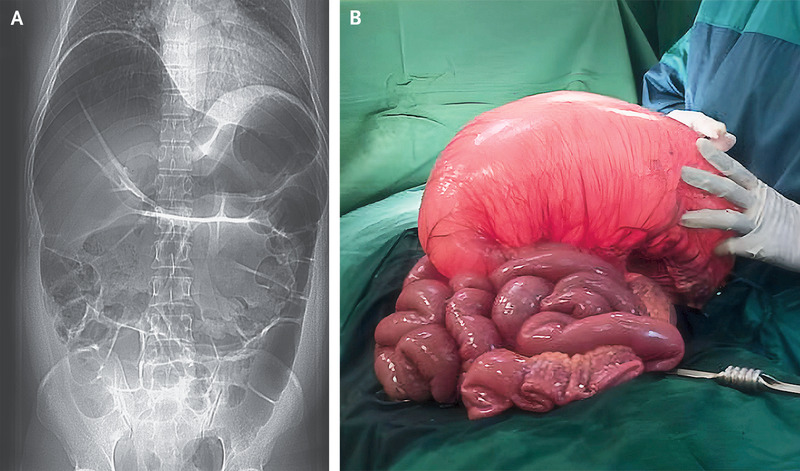
A 37-year-old man presented to the emergency department with an 8-hour history of severe abdominal pain and vomiting. An abdominal radiograph showed dilatation of the large bowel with prominent loops of small bowel (Panel A). Barium enema revealed high-grade obstruction at the splenic flexure. An emergency laparotomy was performed. The transverse colon was found to be massively dilated (Panel B) with severe mucosal edema that extended proximally to the ascending colon. A partial large-bowel resection was performed. After the procedure, the patient reported having had unexplained episodes of abdominal pain and swelling in his hands, feet, and scrotum, without associated hives or itching, since he was 18 years of age. He had never received treatment with an angiotensin-converting-enzyme inhibitor. He reported that his father and paternal relatives had also had similar episodes. Subsequent laboratory testing revealed a low C4 level and a C1 esterase inhibitor level of 6 mg per deciliter (reference range, 19 to 37). A final diagnosis of large-bowel obstruction from gut angioedema in the context of a delayed diagnosis of hereditary angioedema due to C1 esterase inhibitor deficiency was made. Long-term prophylactic treatment with danazol was prescribed, along with C1 esterase inhibitor replacement therapy for recurrent attacks of angioedema. In addition, the patient underwent genetic counselling for this autosomal dominant condition.
(21). Amy H. Farkas et al. Breast Cancer Screening and Prevention. https://doi.org/10.7326/AITC202311210
Breast cancer is the most common cancer among U.S. women and its incidence increases with age. Endogenous estrogen exposure, proliferative benign breast disease, breast density, and family history may also indicate increased risk for breast cancer. Early detection with screening mammography reduces breast cancer mortality, but the net benefits vary by age. Assessing a patient’s individual breast cancer risk can guide decisions regarding breast cancer screening. All women benefit from healthy behaviors which may reduce breast cancer risk. Some women at increased risk for breast cancer may benefit from risk-reducing medications. Use of screening measures remains suboptimal, especially for uninsured women.
(22). Philip Ball et al. Phage stories. The Lancet. 2023;40(10415):1805-1940.
It is hardly surprising that viruses do not have a good press. The very name is derived from the Latin word for a poisonous and perhaps slimy substance. In a medical context, the word originally connoted a putrid excrescence caused by an infectious disease that could transmit the disease to others. The image of viruses as agents apt to spread and cause suffering has been secured in recent times by lethal influenza strains, HIV, and now of course SARS-CoV-2, the coronavirus that stopped the world.
In The Good Virus: The Untold Story of Phages: The Most Abundant Life Forms on Earth and What They Can Do For Us, science journalist Tom Ireland sets out to change that. Some viruses, he explains, help to sustain our health and have saved lives. He is talking about bacteriophages: viruses whose victims are bacteria, including some of the nastiest bacterial pathogens known. In lucid and breezy prose, Ireland explains how phages had a role in the rise of molecular biology, how they shape the biosphere, and how they might be used as alternatives to our ailing antibiotics which are increasingly undermined by antimicrobial resistance.
It has long been recognised that viruses challenge our definitions of life. They were initially thought by many researchers to be mere molecules of some kind. And in a sense that is all they are strands of genetic material packaged in a protein coat, bad news wrapped up in protein, as Peter and Jean Medawar famously put it, sometimes looking more like faceted crystals than living organisms. Many researchers argue that if viruses have any claim to be living at all, it is only when they have infected a host and commandeered its replication machinery. The discovery that viruses are themselves parasitised by pieces of replicating DNA called chromosomal islands only adds to this sense that life appears by degrees and that not all biological agents truly possess it.
Phages were discovered in the late 19th century by the British physician Frederick Twort (1877-1950) and the microbiologist Felix d’Herelle (1873-1949), who claimed to be French-Canadian although his birth certificate was from Paris, France. The two men could not have been more dissimilar: Twort sober and conventional, d’Herelle eccentric and barely scientifically trained. Shortly before World War 1, Twort noticed that some of the bacterial cultures he was preparing would become peppered with holes where the bacteria had apparently been eliminated. Inspection of these voids under the microscope showed nothing but fine granules. He published the findings in a paper in The Lancet in 1915, in which he referred to ultra-microscopic viruses without any clear idea of what they might be perhaps living protoplasm, or some kind of enzyme.
Meanwhile, d’Herelle, having installed himself as a doctor in South America despite having no degree, saw the same phenomenon in colonies of bacteria he was growing to kill locusts. D’Herelle deduced that he had discovered a microbe of microbes, which he named a bacteriophage. With none of Twort’s diffidence, he enthusiastically promoted his idea to the French Academy of Sciences, in the face of intense scepticism from French academics (some of whom seemed chagrined for having missed the phenomenon themselves). One of d’Herelle’s many opponents was delighted to discover that his observations had been anticipated by Twort’s paper, sparking a priority dispute that was never resolved but which might have sunk d’Herelle’s chance of a Nobel Prize he is thought to have been nominated many times.
As the nature of phages became clear, their very simplicity commended them as tools for understanding the chemical basis of life. The first person to fully recognise and exploit that utility was the German physicist-turned-biologist Max Delbr ck, who fled the curdling atmosphere in Germany in 1937 to work at the California Institute of Technology in the USA. For Delbr ck, phages were the atoms of biology; as Ireland puts it: He thought the simplicity of a virus might allow him to work on the fundamental laws and physics of life and reproduction without the complexity of a living organism around it. Working with Italian biologist Salvador Luria in 1943, Delbr ck used the development of bacterial resistance to phages over several generations to deduce that genetic mutations do indeed happen randomly, as Charles Darwin had supposed. At the same time, physician and microbiologist Oswald Avery at the Rockefeller Institute for Medical Research in New York, USA, found evidence that the genetic material was not, as many had suspected, a protein, but rather, the hitherto mysterious DNA. That tentative conclusion was verified in the early 1950s by American microbiologist Alfred Hershey, who along with Delbr ck and Luria completed the Trinity of the Phage Church driving molecular biology forward; the trio were awarded the 1969 Nobel Prize in Physiology or Medicine. With technician Martha Chase, Hershey used an ingenious radioisotope-labelling method to prove that phages replicate by injecting their genetic material into the cells they infect. Trace almost anything being done in a modern molecular biology lab back to its roots, Ireland writes (with only a little exaggeration), and you’ll likely find Delbr ck, Luria or Hershey. And, always, a phage.
Ireland brings this part of the story of phages up to date, exploring their continued role as tools for biotechnology. It is as a defence against phages that some bacteria and archaea have evolved the molecular machinery of the CRISPR genome-editing system. Pieces of viral DNA incorporated into the bacterial genomes from ancestral infection are used to programme the family of Cas enzymes to seek out and cleave identical sequences elsewhere in the cells, disabling phage invaders. The recognition in 2012 by Jennifer Doudna, Emmanuelle Charpentier, and their collaborators that the CRISPR-Cas9 system can be reprogrammed to cut other DNA sequences to order launched it as the genome-editing method of choice that is now promising to transform genetic medicine.
In such ways, Ireland effectively uses a phage-focused lens to recount much of the history of molecular biology from its inception to the present. In addition, his book explores the idea of using phages therapeutically to combat bacterial infection an idea first energetically promoted by d’Herelle. It is a curious, sometimes bizarre and harrowing story. In western countries, interest in phage therapy waned with the advent of antibiotics in the 1930s. But the idea was sustained in the former Soviet Union, where an institute for phage research was established in Tbilisi, Georgia, headed by the mercurial d’Herelle. Those plans collapsed in 1937 when their prime architect, the Georgian microbiologist George Eliava, was arrested by the KGB, largely because of personal animosity with the leader of the Soviet secret police Lavrentiy Beria. Eliava and his wife were executed, although it is not known exactly how or when; as Ireland describes, the Georgian newspaper Kommunisti ran a concocted story about how Eliava was planning to infect wells with deadly bacteria on behalf of the Soviet Union’s enemies.
The facility, now the Eliava Institute, still survives and Ireland gives a compelling first-hand account of its parlous post-independence state. Georgia remains one of the few places in the world where phage medicines can be purchased, but their formulation often an ill-characterised mixture of viruses inhibits acceptance by pharmaceutical regulators elsewhere. Ireland describes how a few individuals with otherwise untreatable bacterial infections have found succour in Georgia’s phage treatments, having made the difficult and often expensive trip to Tbilisi. Ireland reports that between 2012 and 2019, clinics in Tbilisi treated around 10 000 patients, 1500 of them from outside the country. Phage therapy is also offered by the Hirszfeld Institute of Immunology and Experimental Therapy in Poland, albeit with more regulatory restrictions since Poland joined the EU.
But such stories are anecdotal, and phage therapy remains controversial. Since phages cannot be patented, their commercial value to pharmaceutical companies is limited; the former Russian pharmaceutical giant Microgen was one of the few to have sold phage products on a large scale. Here Ireland faces a delicate challenge in explaining the potential of phage therapy without overselling it. The miracle cures found by some people with bacterial infections resistant to all known antibiotics are sometimes dramatic but remain anecdotal. Scepticism about the clinical value of phages is not entirely due to old prejudices: efficacy is hard to show in human trials, and issues such as horizontal gene transfer between phage, microbiome, and human host are troubling. Phage-therapy products are not currently approved for human use in the EU or the USA, although some are permitted in the food industry.
Ireland relates how the range of phages in nature is barely characterised yet but is evidently almost inexhaustible. Several citizen science schemes, such as the SEA-PHAGES (Science Education Alliance-Phage Hunters Advancing Genomics and Evolutionary Science) programme run out of the University of Pittsburgh, PA, USA, and the Citizen Phage Library run by microbiologists in the UK, encourage members of the public to collect samples for testing, or teach students to isolate and sequence the viruses themselves.
Now that entire phages can be synthesised from scratch and their genomes tailored to attack specific bacterial species and strains, there is potential in a phage therapy 2.0, which a few researchers and start-up companies in high-income countries are hoping to develop as antibiotics flounder. Rather than seeking haphazardly for phages that can do the right job, and then having to isolate and purify them, Ireland describes how some think the way ahead is to chemically synthesise bespoke phage genomes on the spot, perhaps loaded with add-on genes for breaking down bacterial biofilms, or for programming a given survival time, or tailored to the patient’s own genome. That remains a distant dream, but such technologies could potentially give phage therapy the precise specification of conventional molecular drugs. It remains to be seen if the best is indeed yet to come for medical uses of phages, but The Good Virus offers an excellent overview of the current state of play.
(23). Erin E West et al. Complement in human disease: approved and up-and-coming therapeutics. https://doi.org/10.1016/S0140-6736(23)01524-6
The complement system is recognised as a protector against blood-borne pathogens and a controller of immune system and tissue homoeostasis. However, dysregulated complement activity is associated with unwanted or non-resolving immune responses and inflammation, which induce or exacerbate the pathogenesis of a broad range of inflammatory and autoimmune diseases. Although the merit of targeting complement clinically has long been acknowledged, the overall complement drug approval rate has been modest. However, the success of the humanised anti-C5 antibody eculizumab in effectively treating paroxysmal nocturnal haemoglobinuria and atypical haemolytic syndrome has revitalised efforts to target complement therapeutically. Increased understanding of complement biology has led to the identification of novel targets for drug development that, in combination with advances in drug discovery and development technologies, has resulted in a surge of interest in bringing new complement therapeutics into clinical use. The rising number of approved drugs still almost exclusively target rare diseases, but the substantial pipeline of up-and-coming treatment options will possibly provide opportunities to also expand the clinical targeting of complement to common diseases
(24). Prof David W Dodick et al. Ubrogepant for the treatment of migraine attacks during the prodrome: a phase 3, multicentre, randomised, double-blind, placebo-controlled, crossover trial. https://doi.org/10.1016/S0140-6736(23)01683-5
Background
Ubrogepant is a calcitonin gene-related peptide (CGRP) receptor antagonist that is approved for acute treatment of migraine. The prodrome is the earliest phase of a migraine attack and is characterised by non-aura symptoms that precede headache onset. The aim of this trial was to evaluate the efficacy, safety, and tolerability of ubrogepant 100 mg compared with placebo for the acute treatment of migraine when administered during the prodrome.
Methods
This PRODROME trial was a phase 3, multicentre, randomised, double-blind, placebo-controlled, crossover trial of ubrogepant 100 mg conducted at 75 research centres and headache clinics in the USA. Eligible participants were adults aged 18-75 years who had at least a 1-year history of migraine with or without aura and a history of two to eight migraine attacks per month with moderate to severe headache in each of the 3 months before screening. Eligible participants were randomly assigned (1:1) to either receive placebo to treat the first qualifying prodrome event and ubrogepant 100 mg to treat the second qualifying prodrome event or to receive ubrogepant 100 mg to treat the first qualifying prodrome event and placebo to treat the second qualifying prodrome event. An automated interactive web-response system used permuted blocks of four to manage randomisation. All people giving interventions and assessing outcomes were masked to group assignment during the study. People doing data analysis, which occurred after study completion, were not masked to group assignment. During the double-blind treatment period, each participant was instructed to orally take two tablets of the study drug at the onset of each qualifying prodrome event. The primary endpoint was absence of moderate or severe intensity headache within 24 h after study-drug dose; efficacy analyses were conducted with the modified intention-to-treat (mITT) population, defined as all randomly assigned participants with at least one headache assessment within 24 h after taking the study drug during the treatment period. The safety population included all treated participants who took at least one administration of study drug. The trial is registered with ClinicalTrials.gov (NCT04492020).
Findings
Between Aug 21, 2020, and April 19, 2022, 518 participants were randomly assigned to double-blind crossover treatment. The safety population included 480 participants and the mITT population included 477 participants; 421 (88%) of 480 participants were female and 59 (12%) were male. Absence of moderate or severe headache within 24 h after a dose occurred after 190 (46%) of 418 qualifying prodrome events that had been treated with ubrogepant and after 121 (29%) of 423 qualifying prodrome events that had been treated with placebo (odds ratio 2.09, 95% CI 1.63-2.69; p-0.0001). Adverse events that occurred within 48 h after study-drug administration were reported after 77 (17%) of 456 qualifying prodrome events that had been treated with ubrogepant and after 55 (12%) of 462 events that had been treated with placebo.
Interpretation
Ubrogepant was effective and well tolerated for the treatment of migraine attacks when taken during the prodrome.
(25). Elisabeth Mahase et al. Cardiac arrest occurs in three in 10 000 surgeries involving anaesthesia, audit finds. BMJ. 2023; 383:2737.
Cardiac arrests occur in around three in 10,000 surgeries requiring anaesthesia, with this number dropping to one in 10,000 in healthy patients undergoing routine surgery, the Royal College of Anaesthetists has reported.
The college’s seventh national audit examined all cardiac arrests occurring during or soon after surgery in more than 300 UK hospitals between June 2021 and June 2022. The research team reported 881 cases among a caseload of 2.71 million, giving an incidence of three per 10,000.
They found that the most common cause was major bleeding (17%).
(26). Mark Thomaz Ugliara Baron et al. Sleep disorders are an overlooked risk factor for non-communicable diseases. BMJ. 2023;383:2721.
Ignoring sleep disorders will prevent reduction of premature mortality from non-communicable diseases.
Sleep disorders such as sleep apnoea, insomnia, and short sleep duration have long been identified as risk factors for the development and exacerbation of NCDs and mental disorder27.
In the past two decades, our understanding of the associations between sleep disorders and NCDs has grown. People with chronic insomnia lasting more than eight years had a 21% higher risk of hypertension and 51% increased risk of type 2 diabetes. Moderate to severe sleep apnoea is associated with a 63% greater risk of type 2 diabetes and 30% higher risk of hypertension.
Short sleep duration impairs people’s quality of life and health. It increases people’s risk of type 2 diabetes, hypertension, cardiovascular diseases, coronary heart diseases, obesity, and strokes.The reversal of sleep and wake times, common in shift and night work, can also increase the risk of NCDs and their exacerbation. Misalignment between the sleep-wake cycle and circadian timing system caused by this type of work schedule has been identified as a risk factor for breast, prostate, and colorectal cancer.
Despite their negative consequences, sleep disorders are often underdiagnosed and undertreated.
(27). Mark Thursz, et al. Advances in the understanding and management of alcohol-related liver disease. BMJ. 2023;383:e077090.
Alcohol-related liver disease (ALD) is a major cause of liver-related morbidity and mortality. Epidemiological trends indicate recent and predicted increases in the burden of disease. Disease progression is driven by continued alcohol exposure on a background of genetic predisposition together with environmental cofactors. Most individuals present with advanced disease despite a long history of excessive alcohol consumption and multiple missed opportunities to intervene. Increasing evidence supports the use of non-invasive tests to screen for and identify disease at earlier stages. There is a definite role for public health measures to reduce the overall burden of disease. At an individual level, however, the ability to influence subsequent disease course by modifying alcohol consumption or the underlying pathogenic mechanisms remains limited due to a comparative lack of effective, disease-modifying medical interventions. Abstinence from alcohol is the key determinant of outcome in established ALD and the cornerstone of clinical management. In those with decompensated ALD, liver transplant has a clear role. There is consensus that abstinence from alcohol for an arbitrary period should not be the sole determinant in a decision to transplant. An increasing understanding of the mechanisms by which alcohol causes liver disease in susceptible individuals offers the prospect of new therapeutic targets for disease-modifying drugs. Successful translation will require significant public and private investment in a disease area which has traditionally been underfunded when compared to its overall prevalence.
(28). Danny Jenkins et al. Proton pump inhibitors. BMJ. 2023;383:e070752.
What do you need to know?
Discuss with patients the importance of lifestyle modification for the long-term management of acid reflux disorders
Discuss with patients that a prescription may be stopped even if it has improved symptoms
Side effects of PPIs include gastrointestinal disturbance, higher rate of fractures, and increased susceptibility to Clostridium difficile infection
Review new prescriptions of proton pump inhibitors (PPIs) at four weeks, and regularly thereafter, assessing for improvement in symptoms and quality of life
A 42-year-old man visits his general practitioner with a three-month history of intermittent nausea and central chest discomfort, particularly after fatty and sweet meals. He occasionally has a bad taste in his mouth when he wakes up from sleep. His body mass index is 32, he has no significant medical history, and he takes no regular medications. After asking him about alarm symptoms, you make a diagnosis of gastro-oesophageal reflux disease. You are considering prescribing a proton pump inhibitor as a first-line therapy.
How often are proton pump inhibitors prescribed and how do they work?
Prevalence of proton pump inhibitor (PPI) use is about 15% of the UK population, according to two primary care database studies. In 2022-23, 73 million NHS prescriptions for PPIs were dispensed in England at a cost of about 190 million, equating to 1.8% of total primary care prescription costs and 6% of all prescriptions dispensed. The two most commonly prescribed PPIs in England are omeprazole and lansoprazole, which were among the five most commonly dispensed drugs in 2020.
There is strong evidence to support the use of PPIs across a range of indications. PPIs are used in the treatment of upper gastrointestinal disorders characterised by excessive acid production and damage to upper gastrointestinal tissue and are also prescribed as gastroprotection for patients being treated with medications which increase the risk of ulceration, such as NSAID.
(29). Rion Healy et al. Chronic prostatitis (chronic pelvic pain syndrome). BMJ. 2023. 383:e073908.
What do you need to know?
Chronic prostatitis, also known as chronic pelvic pain syndrome, is defined as pelvic pain with variable associated urinary symptoms and sexual dysfunction for at least three months
Explore important differentials such as urological and rectal cancer, bacterial infection, benign prostatic enlargement, and neurological conditions
Empower patients with knowledge about the condition and consider psychosocial and quality of life issues early on
A 64-year-old man presents with recurrent episodes of perineal and testicular pain associated with urinary frequency. He was previously treated with antibiotics for prostatitis which didn’t help. He is very concerned about what these symptoms could signal and why they keep coming back.
CPPS has a prevalence of 2-10% of men, according to literature reviews of population-based studies from North America, Finland, Singapore, Japan, and Malaysia. A large cross-sectional study suggests the risk increases with age and is defined as pelvic pain with variable associated urinary symptoms and sexual dysfunction that occurs for at least three months. A common condition can affect a patient’s quality of life. The National Institutes of Health have defined four categories of prostatitis (table 1). Type 3, chronic non-bacterial prostatitis, is the most common, and accounts for almost 90% of all diagnoses. Chronic prostatitis is also known as chronic pelvic pain syndrome (CPPS) or primary prostate pain syndrome. Recently, bodies such as the European Association of Urology (EAU) are trying to move away from the term chronic prostatitis, as CPPS should be considered separate from acute and chronic bacterial prostatitis and is not associated with active infection.
-CPPS has a prevalence of 2-10% of men, according to literature reviews of population based studies from North America, Finland, Singapore, Japan, and Malaysia. A large cross-sectional study suggests the risk increases with age.
(30). Thomas M Caparrotta, et al. Paracetamol use in adults. BMJ. 2023;383:e070753.
What do you need to know?
Short-term use of paracetamol can be effective for acute pain but is unlikely to offer relief for chronic pain
Consider adjusting dose or dose frequency for patients with low body weight, who consume alcohol regularly, have liver disease, or who are frail
Overdose resulting in liver damage can occur in patients who accidentally take too much paracetamol or multiple paracetamol-containing products
Practical Prescribing is a series produced in conjunction with the Drug and Therapeutics Bulletin to highlight important issues for prescribers to consider and prompts for shared decision-making between prescribers, patients, and their carers. Targeted at all medical and non-medical prescribers, particularly doctors in training, the series covers medicines commonly prescribed in primary and secondary care.
A 75-year-old woman has knee pain owing to severe osteoarthritis. She is using topical ibuprofen gel and is taking indapamide and ramipril as antihypertensive medicines. She weighs 49 kg. She has asked whether she should be taking paracetamol regularly.
How often is paracetamol prescribed and how does it work?
Paracetamol is widely available over the counter as a single agent and in multiple combination products with other analgesics such as codeine, tramadol, and ibuprofen. Despite its over-the-counter availability, it is the most commonly prescribed analgesic worldwide. In England, 16 million prescriptions were dispensed from April 2022 to March 2023, and in Scotland, more than 500,000 patients received three or more primary care prescriptions for medications that contained paracetamol in 2018.2 Typical licensed indications for paracetamol in adults include treatment of the most painful and febrile conditions, including headache, neuralgia, sore throat, and rheumatic pain.3.
Paracetamol inhibits the enzyme cyclooxygenase (COX), which produces pro-inflammatory prostaglandins and thromboxane (fig 1). Non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen have a similar mechanism of action. Uncertainty exists as to whether paracetamol should be classified as different to NSAID.
