Journal scan
A review of 20 recent papers of immediate clinical significance, harvested from major international journals
From the desk of the Editor-in-Chief
(1). Charlotte Debras, et al. Artificial sweeteners and risk of cardiovascular diseases: results from the prospective NutriNet-Santé cohort. BMJ 2022;378:e071204
Abstract
Objectives: To study the associations between artificial sweeteners from all dietary sources (beverages, but also table-top sweeteners, dairy products, etc), overall and by molecule (aspartame, acesulfame potassium, and sucralose), and risk of cardiovascular diseases (overall, coronary heart disease, and cerebrovascular disease).
Design: Population-based prospective cohort study (2009-21).
Setting: France, primary prevention research.
Participants: 103 388 participants of the web-based NutriNet-Santé cohort (mean age 42.2±14.4, 79.8% female, 904 206 person-years). Dietary intakes and consumption of artificial sweeteners were assessed by repeated 24 h dietary records, including brand names of industrial products.
Main outcomes measures: Associations between sweeteners (coded as a continuous variable, log10 transformed) and cardiovascular disease risk, assessed by multivariable adjusted Cox hazard models.
Results: Total artificial sweetener intake was associated with increased risk of cardiovascular diseases (1502 events, hazard ratio 1.09, 95% confidence interval 1.01 to 1.18, P=0.03); absolute incidence rate in higher consumers (above the sex specific median) and non-consumers was 346 and 314 per 100 000 person years, respectively. Artificial sweeteners were more particularly associated with cerebrovascular disease risk (777 events, 1.18, 1.06 to 1.31, P=0.002; incidence rates 195 and 150 per 100 000 person years in higher and non-consumers, respectively). Aspartame intake was associated with increased risk of cerebrovascular events (1.17, 1.03 to 1.33, P=0.02; incidence rates 186 and 151 per 100 000 person years in higher and non-consumers, respectively), and acesulfame potassium and sucralose were associated with increased coronary heart disease risk (730 events; acesulfame potassium: 1.40, 1.06 to 1.84, P=0.02; incidence rates 167 and 164; sucralose: 1.31, 1.00 to 1.71, P=0.05; incidence rates 271 and 161).
Conclusions: The findings from this large-scale prospective cohort study suggest a potential direct association between higher artificial sweetener consumption (especially aspartame, acesulfame potassium, and sucralose) and increased cardiovascular disease risk. Artificial sweeteners are present in thousands of food and beverage brands worldwide, however, they remain a controversial topic and are currently being re-evaluated by the European Food Safety Authority, the World Health Organization, and other health agencies.
(2). Karina Ferreira et al. Vestibulotoxicity with gentamicin. BMJ 2022;378:e070873.
What you need to know
Ask for a history of gentamicin administration in patients who experience vestibular symptoms such as imbalance while walking or wobbly vision
Gentamicin related vestibulotoxicity is often permanent, but prompt diagnosis and early physical rehabilitation can improve gait and balance
Avoid gentamicin, if possible, for surgical prophylaxis and in patients with risk factors such as pre-existing kidney disease, overweight, or using other drugs that can potentiate its effects (such as vancomycin)
A patient reports feeling unsteady while walking. This has come up suddenly. He had a renal stent inserted for calculi removal two days previously, for which he received intravenous gentamicin as prophylaxis. He also reports wobbly vision on head movement, which resolves with his head held still. Neurological examination reveals severe gait imbalance, with the patient requiring support for walking even short distances. He is diagnosed with gentamicin related vestibulotoxicity and started on vestibular rehabilitation.
Aminoglycoside antibiotics are implicated in functional impairment and/or cellular damage to the vestibular system as an adverse reaction (box 1).4 Gentamicin is the most frequently used aminoglycoside antibiotic in adults. As with other aminoglycosides such as streptomycin and tobramycin, gentamicin related vestibulotoxicity is typically bilateral due to systemic administration. The damage can be permanent. Early diagnosis can help initiate measures to improve balance. This article focuses on gentamicin related vestibulotoxicity in adults. Symptoms and the indications for gentamicin use, dosing, and monitoring can differ in children.
Safety warnings for gentamicin and aminoglycosides
The British National Formulary (BNF) advises caution regarding “vestibular disorder,” and avoidance of “prolonged” use1
The Electronic Medicines Compendium summary of product characteristics lists a special warning on ototoxicty (loss of balance and hearing loss) with use of aminoglycosides, including gentamicin2
The US Food and Drug Administration advises that ototoxicity, both vestibular and auditory, can occur in patients treated with gentamicin
(3). Mercedes J. Burnside, et al. Open-Source Automated Insulin Delivery in Type 1 Diabetes. N Engl J Med. 2022;387:869-881
Background
Open-source automated insulin delivery (AID) systems are used by many patients with type 1 diabetes. Data are needed on the efficacy and safety of an open-source AID system.
Methods
In this multicenter, open-label, randomized, controlled trial, we assigned patients with type 1 diabetes in a 1:1 ratio to use an open-source AID system or a sensor-augmented insulin pump (control). The patients included both children (defined as 7 to 15 years of age) and adults (defined as 16 to 70 years of age). The AID system was a modified version of AndroidAPS 2.8 (with a standard OpenAPS 0.7.0 algorithm) paired with a preproduction DANA-i insulin pump and Dexcom G6 CGM, which has an Android smartphone application as the user interface. The primary outcome was the percentage of time in the target glucose range of 70 to 180 mg per deciliter (3.9 to 10.0 mmol per liter) between days 155 and 168 (the final 2 weeks of the trial).
Results
A total of 97 patients (48 children and 49 adults) underwent randomization (44 to open-source AID and 53 to the control group). At 24 weeks, the mean (±SD) time in the target range increased from 61.2±12.3% to 71.2±12.1% in the AID group and decreased from 57.7±14.3% to 54.5±16.0% in the control group (adjusted difference, 14 percentage points; 95% confidence interval, 9.2 to 18.8; P<0.001), with no treatment effect according to age (P=0.56). Patients in the AID group spent 3 hours 21 minutes more in the target range per day than those in the control group. No severe hypoglycemia or diabetic ketoacidosis occurred in either group. Two patients in the AID group withdrew from the trial owing to connectivity issues.
Conclusions
In children and adults with type 1 diabetes, the use of an open-source AID system resulted in a significantly higher percentage of time in the target glucose range than the use of a sensor-augmented insulin pump at 24 weeks.
(4). Richard A. Furie et al. Trial of Anti-BDCA2 Antibody Litifilimab for Systemic Lupus Erythematosus. N Engl J Med. 2022;387:894-904.
Background
Antibody-binding of blood dendritic cell antigen 2 (BDCA2), which is expressed exclusively on plasmacytoid dendritic cells, suppresses the production of type I interferon that is involved in the pathogenesis of systemic lupus erythematosus (SLE). The safety and efficacy of subcutaneous litifilimab, a humanized monoclonal antibody that binds to BDCA2, in patients with SLE have not been extensively studied.
Methods
We conducted a phase 2 trial of litifilimab involving participants with SLE. The initial trial design called for randomly assigning participants to receive litifilimab (at a dose of 50, 150, or 450 mg) or placebo administered subcutaneously at weeks 0, 2, 4, 8, 12, 16, and 20, with the primary end point of evaluating cutaneous lupus activity. The trial design was subsequently modified; adults with SLE, arthritis, and active skin disease were randomly assigned to receive either litifilimab at a dose of 450 mg or placebo. The revised primary end point was the change from baseline in the total number of active joints (defined as the sum of the swollen joints and the tender joints) at week 24. Secondary end points were changes in cutaneous and global disease activity. Safety was also assessed.
Results
A total of 334 adults were assessed for eligibility, and 132 underwent randomization (64 were assigned to receive 450-mg litifilimab, 6 to receive 150-mg litifilimab, 6 to receive 50-mg litifilimab, and 56 to receive placebo). The primary analysis was conducted in the 102 participants who had received 450-mg litifilimab or placebo and had at least four tender and at least four swollen joints. The mean (±SD) baseline number of active joints was 19.0±8.4 in the litifilimab group and 21.6±8.5 in the placebo group. The least-squares mean (±SE) change from baseline to week 24 in the total number of active joints was -15.0±1.2 with litifilimab and –11.6±1.3 with placebo (mean difference, -3.4; 95% confidence interval, -6.7 to -0.2; P=0.04). Most of the secondary end points did not support the results of the analysis of the primary end point. Receipt of litifilimab was associated with adverse events, including two cases of herpes zoster and one case of herpes keratitis.
Conclusions
In a phase 2 trial involving participants with SLE, litifilimab was associated with a greater reduction from baseline in the number of swollen and tender joints than placebo over a period of 24 weeks. Longer and larger trials are required to determine the safety and efficacy of litifilimab for the treatment of SLE
(5). Charlotte K. Boughton et al. Closed-Loop Therapy and Preservation of C-Peptide Secretion in Type 1 Diabetes. N Engl J Med. 2022;387:882-893..
Background
Whether improved glucose control with hybrid closed-loop therapy can preserve C-peptide secretion as compared with standard insulin therapy in persons with new-onset type 1 diabetes is unclear.
Methods
In a multicenter, open-label, parallel-group, randomized trial, we assigned youths 10.0 to 16.9 years of age within 21 days after a diagnosis of type 1 diabetes to receive hybrid closed-loop therapy or standard insulin therapy (control) for 24 months. The primary end point was the area under the curve (AUC) for the plasma C-peptide level (after a mixed-meal tolerance test) at 12 months after diagnosis. The analysis was performed on an intention-to-treat basis.
Results
A total of 97 participants (mean [±SD] age, 12±2 years) underwent randomization: 51 were assigned to receive closed-loop therapy and 46 to receive control therapy. The AUC for the C-peptide level at 12 months (primary end point) did not differ significantly between the two groups (geometric mean, 0.35 pmol per milliliter [interquartile range, 0.16 to 0.49] with closed-loop therapy and 0.46 pmol per milliliter [interquartile range, 0.22 to 0.69] with control therapy; mean adjusted difference, −0.06 pmol per milliliter [95% confidence interval {CI}, −0.14 to 0.03]). There was not a substantial between-group difference in the AUC for the C-peptide level at 24 months (geometric mean, 0.18 pmol per milliliter [interquartile range, 0.06 to 0.22] with closed-loop therapy and 0.24 pmol per milliliter [interquartile range, 0.05 to 0.30] with control therapy; mean adjusted difference, −0.04 pmol per milliliter [95% CI, −0.14 to 0.06]). The arithmetic mean glycated hemoglobin level was lower in the closed-loop group than in the control group by 4 mmol per mole (0.4 percentage points; 95% CI, 0 to 8 mmol per mole [0.0 to 0.7 percentage points]) at 12 months and by 11 mmol per mole (1.0 percentage points; 95% CI, 7 to 15 mmol per mole [0.5 to 1.5 percentage points]) at 24 months. Five cases of severe hypoglycemia occurred in the closed-loop group (in 3 participants), and one occurred in the control group; one case of diabetic ketoacidosis occurred in the closed-loop group.
Conclusions
In youths with new-onset type 1 diabetes, intensive glucose control for 24 months did not appear to prevent the decline in residual C-peptide secretion
(6). Neil Osterweil. Obstructive Sleep Apnea Linked to Unprovoked VTE.
https://www.medscape.com/viewarticle/980453
BARCELONA, Spain – Add unprovoked venous thromboembolic events to the list of potential consequences of severe obstructive sleep apnea.
That conclusion comes from a study showing that patients with obstructive sleep apnea (OSA) who had the longest nocturnal hypoxemia episodes had a twofold risk for venous thromboembolic events.
The association between nocturnal hypoxemia and VTE was strongest among patients who did not use continuous positive airway pressure (CPAP) systems, reported Wojciech Trzepizur, MD, from Angers University Hospital in Angers, France.
Previous studies have suggested links between OSA and both cancer and cognitive decline, but this is the first study to investigate the association between OSA and the incidence of unprovoked VTE, he reported in an oral abstract session at the European Respiratory Society 2022 Congress.
“We found that those who spent more than 6% of their nighttime with levels of oxygen in their blood below 90% of normal had an almost twofold risk of developing VTEs as compared to patients without oxygen deprivation,” he said. Trzepizur and colleagues conducted a retrospective study linking cohort data to an administrative health database. They identified unprovoked VTE in patients with a suspicion for OSA and no previous VTE.
They created Cox proportional hazard models to assess the association of unprovoked VTE with apnea hypopnea index (AHI) measures and nocturnal hypoxemia markers, including the time patients spent below 90% oxygen saturation (T90), oxygen desaturation index (ODI), and hypoxic burden, defined as the total area under the respiratory event-related desaturation curve.
They found that after a median follow-up of 6.3 years, 104 out of 7355 patients had an unprovoked VTE. In an unadjusted hazard model, there were significant associations between VTE and T90, as well as with hypoxic burden, but not with either AHI or ODI.
However, in an analysis adjusted for age, gender, body mass index, alcohol intake, hypertension, depression, history of cardiovascular disease, statin use, type of sleep study, study site and CPAP adherence, the investigators found that only T90 remained a significant independent predictor of VTE, with a hazard ratio (HR) of 1.06, P = .02.
The association between T90 and VTE strengthened as the time spent below 90% saturation increased. Patients in the highest tercile, who spent more than 6% of the time undersaturated, had an HR for VTE of 1.95 (P = .02) compared with patients with a T90 less than 1%.
There were no significant differences in VTE risk between patients who used CPAP for more than 4 hours per night compared with those who either used the devices for less than 4 hours or refused CPAP.
Session co-moderator Raphael Heinzer, MD, MPH, Lausanne University Hospital, Lausanne, Switzerland, commented, “We see that T90 seems to be a strong parameter.”
Heinzer’s co-moderator Silke Ryan, MD, from University College Dublin, Ireland, pointed out that although T90 was the main predictor of responses, Trzepizur and colleagues did not control for other pulmonary diseases.
“Obviously, there could be an influence of other hypoxic-related diseases,” she said, and recommended controlling for this in future studies.
Professor Winfried Randerath, MD, of the Bethanien Hospital at the University of Cologne, Germany, head of the ERS specialist group on sleep disordered breathing, commented that this study and others presented at the meeting “show worrying associations between obstructive sleep apnea and important diseases that affect survival and quality of life.”
“While they cannot prove that OSA causes any of these health problems, people should be made aware of these links and should try to make lifestyle changes in order to reduce their risk of OSA, for instance, by maintaining a healthy weight. However, if OSA is suspected, definite diagnosis and treatment should be initiated. We look forward to further research that may help to clarify whether OSA may be causing some of the health problems seen in these studies,” said Randerath, who was not involved with the study.
(7). Jocelyn McCullough. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2022;387:e20.
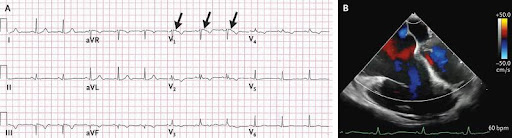
A 59-year-old woman was admitted to the hospital with a subdural hematoma after an episode of unheralded syncope. She had lost a son to sudden cardiac death when he was 29 years of age. On hospital day 2, the patient began to have sustained monomorphic ventricular tachycardia with a pulse. Synchronized cardioversion was performed, and treatment with intravenous amiodarone was initiated. After the event, an electrocardiogram showed an incomplete right bundle-branch block, T-wave inversions in leads V1 through V4, and an epsilon wave in lead V1 (Panel A, arrows). A transesophageal echocardiogram showed a severely dilated right ventricle (Panel B and video). Cardiac magnetic resonance imaging showed a right ventricular ejection fraction of 27% with regional akinesis. A diagnosis of arrhythmogenic right ventricular cardiomyopathy was made. An implantable cardioverter-defibrillator was placed, and treatment with metoprolol and antiarrhythmic medication was initiated. Genetic testing was performed, but a known pathogenic variant was not found. The patient was advised to avoid strenuous exercise, and cardiac evaluation was recommended for her first-degree relatives. On follow-up echocardiography 3 months later, the patient had a reduced left ventricular ejection fraction of 40%. Owing to the development of biventricular heart failure, she underwent evaluation for cardiac transplantation and currently awaits a heart transplant.
(8). Loren E Hernandez. Human monkeypox virus infection in an immunocompromised man: trial with tecovirimat. 2022;400(10355):E8.
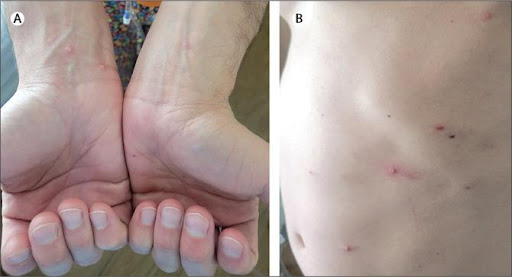
A 37-year-old man attended our hospital with a 1-week history of fever, chills, headaches, sore throat, generalised malaise, and a rash on his arms, legs, trunk, and in his groin. The patient also reported significant pain and discomfort in his rectum when defecating. He had a history of HIV and metastatic Kaposi sarcoma; secondary syphilis, which had been treated; and hypertension. He was prescribed the following medications: emtricitabine-tenofovir, doravarine, darunavir-cobicistat, and hydrochlorothiazide.
The patient reported no recent travel or contact with human or animal with a similar rash or illnesses. He reported no history of varicella zoster virus infection as a child and said he had received all childhood vaccinations. The patient reported no sexual activity for the past 6 months.
On examination, the patient was afebrile: temperature 37.3°C. He appeared uncomfortable and had several scattered, pink, umbilicated papules, vesicles, and pustules on the trunk, upper and lower extremities, groin, and peri-anal region.
Laboratory investigations showed a haemoglobin concentration of 12.7 g/dL (normal 13.3-16.3), CD4+ T-helper cell count of 262 cells per mcL (normal 490-1740), HIV-1 RNA PCR less than 20 copies per mL, and an absolute lymphocyte count of 3.01 per uL (normal 1.1-2.7). Serology was negative for Bartonella henselae, Aspergillus spp, hepatitis A, B, and C, cryptococcal antigen, herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), and (1→3)-β-D-glucan.
Immunohistochemical analysis of a biopsy sample of one of the truncal papules was negative for HSV-1, HSV-2, and Treponema pallidum. Warthin-Starry staining was negative for spirochetes and periodic-acid-Schiff staining was negative for fungal organisms. Histopathological analysis of the biopsy sample showed focal epidermal necrosis with underlying acute inflammation in the superficial dermis and individually necrotic keratinocytes at the periphery. A Dacron swab of one of the truncal lesions was positive for human monkeypox virus.
Considering our patient’s history of immunosuppression, we prescribed doxycycline, ceftriaxone, and valacyclovir; we subsequently gave him a 2-week course of 600 mg of tecovirimat twice a day. He reported no significant adverse effects and the skin lesions healed rapidly. At follow-up one month later, the patient reported areas of hyperpigmentation at the sites of the healed lesions and said he was doing well.
Monkeypox is caused by the monkeypox virus, a member of the Poxviridae family and Orthopoxvirus genus. The virus is transmitted via large respiratory droplets, direct or indirect contact with bodily fluids or lesion material, or contact with fomites-including bedding or towels. Malaise, lymphadenopathy, headache, myalgia, and cutaneous symptoms can occur within 1-3 days of the onset of fever. Lesions may initially appear as macules progressing to papules, vesicles, and pustules that dry up and fall off. The development of skin lesions, in addition to viral prodromal symptoms, in immunocompromised patients should raise the possibility of other diagnosis, such as primary or secondary infection with varicella zoster virus or other organisms such as Cryptococcus neoformans, Histoplasma capsulatum, or Bartonella henselae; we considered this unlikely in our patient given his presentation. Molluscum contagiosum also presents with umbilicated papules, but usually without systemic symptoms. Notably, proctitis has recently been recognised as being associated with a human monkeypox infection. Tecovirimat is approved for treating human smallpox disease. Treatment in cases of human monkeypox should be considered in severe cases, immunocompromised patients, paediatric populations, pregnant or breastfeeding women, and people with concurrent disease or other comorbidities.
(9). Image Challenge. The NEJM 2022
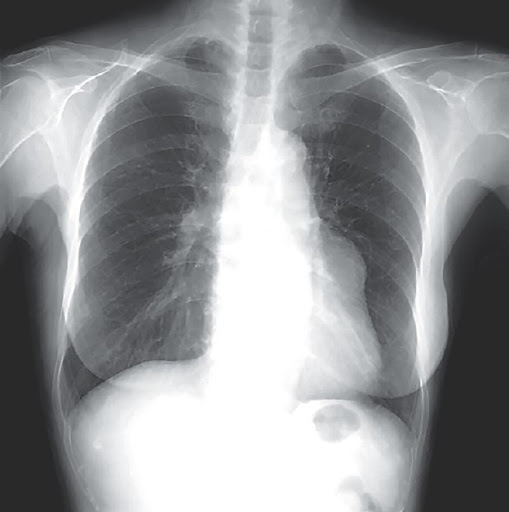
A 79-year-old woman with a history of prior stroke was referred for an abnormal X-ray finding along the left heart border, first noticed 6 years prior. In the absence of symptoms, the patient was monitored with serial radiographs which showed a gradual increase in size of the finding. What is the most likely diagnosis?
Answer: Giant Coronary Aneurysm!
(10). Hans Christoph Diener, et al. Antithrombotic drugs in secondary stroke prevention: still some way to go. Lancet. 2022;400(10357):P974-975.
Patients who have ischaemic stroke or a transient ischaemic attack have a high risk of recurrent ischaemic stroke. This risk is highest in the first few days after the primary ischaemic event and then slowly decreases over time. Therefore, it is important to start secondary prevention as soon as possible, ideally oriented to the pathophysiology of cerebral ischaemia in the individual patient. Early antithrombotic therapy is particularly important. Several large randomised trials have found that aspirin significantly reduces the risk of recurrent ischaemic stroke in patients without overt atrial fibrillation, with the greatest benefit in the first few weeks after transient ischaemic attack or stroke. 1 Subsequent trials found that the combinations of aspirin plus clopidogrel or aspirin plus ticagrelor were more effective than aspirin monotherapy during this early high-risk period, albeit with an increased bleeding risk. 2 However, in longer-term secondary prevention, combination of antiplatelet agents such as aspirin plus clopidogrel were not more effective than monotherapy with aspirin or clopidogrel. 2 Therefore, longer-term secondary prevention usually focuses on treatment of major risk factors such as arterial hypertension, hyperlipidaemia, and diabetes as well as lifestyle modifications such as regular physical activity, weight loss, diet, avoidance of nicotine, and reduction of alcohol consumption. 3 These secondary prevention strategies each yield an absolute reduction in risk of stroke of 0.2% to 0.4% per year. In patients with atrial fibrillation, the use of oral anticoagulants is also highly effective in preventing recurrent stroke, with an absolute risk reduction of 7.3% per year with vitamin K antagonists and similar benefits with non-vitamin K oral anticoagulants, which also have a lower bleeding risk than vitamin K antagonists.
(11). John Launer. The art of paying attention. BMJ 2022;378:o2294
I’m not sure that I’ve ever been an exceptionally compassionate or empathetic doctor, and it would be for patients to say, rather than me. Possibly they’d have a range of views, depending on whether we got on well or my treatment made them better. Altogether, I’m a bit suspicious of training and publications that describe how to develop empathy and compassion (sometimes subtly implying saintliness in their authors), so in my own teaching I generally try to avoid tackling these topics head on.
There are other qualities I prefer to focus on. Rita Charon, a New York physician and a pioneer in the field of narrative medicine, emphasises three qualities that she believes we should promote in medicine: attention, representation, and affiliation.1 She describes attention as “the most pivotal skill with which to endow a health professional who wants to be a healer.” By representation she means the capacity to give a faithful and accurate account of a patient’s words, images, thoughts, and utterances. Affiliation follows from these, when we bring our full selves into our practice.
When I’m teaching I like to focus on attention, although I usually prefer the term “attentiveness,” as it implies something more continuous. It highlights the idea that defines narrative medicine: you can apply close reading to a patient’s story in the same way you can to a literary text. Attentiveness carries nuances of both empathy and compassion, without the risk of judgmental overtones. Most important of all, it can be translated into a set of skills for careful questioning, listening, and responding that you can readily teach and learn.
Among the skills for attentiveness that I find most useful to teach are “noticing the words we usually ignore” and “taking the temperature of the conversation.” Regarding the first of these, I’m always interested in statements such as, “My headaches are driving me to despair.” Ninety-nine times in 100 we ask about the headaches and never inquire about the “driving to despair.” Why did that person, consciously or unconsciously, select that exact phrase-and what more will we learn about their life, and possibly even the cause of the headaches, if we’re curious about this and ask them to unpack it?
The other skill involves what are sometimes called “meta-questions.” These are questions such as: “What are you hoping to get from this consultation?” “How are we doing so far?” “Have I missed out anything you asked?” “Is it OK to wind up now?” Almost every time I teach these and other similar skills, someone is almost bound to object that it will be unnecessarily time consuming. Yet almost everyone who applies them reports that it helps them get to the heart of things in consultations far more quickly.
The US physician and ethicist Jay Katz once criticised the “dogma” in medicine of “assuming an identity of interests and brushing aside the need to clarify differences in expectations and objectives through conversation.”2 It’s one of my favourite quotations. The antidote to this dogma is attentiveness. And whenever we apply it, I suspect that many patients experience it as empathy and compassion, even if we don’t think of it as such.
(12). JunQing Xie, et al. Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients With COVID-19. JAMA Intern Med. 2022;182(10):1063-1070.
Question: What is the 30-day acute risk of venous thromboembolism (VTE) among ambulatory patients with COVID-19, and what are the clinical and genetic risk factors predisposing them to developing post-COVID-19 VTE?
Findings: In this retrospective cohort study of 18,818 outpatients with COVID-19 and 93,179 propensity score-matched noninfected participants, a higher VTE incidence was observed in the former (hazard ratio, 21.42); however, this risk was considerably attenuated among the fully vaccinated, after breakthrough infection. Older age, male sex, obesity, no vaccination or partial vaccination, and inherited thrombophilia were independent risk factors for COVID-19-associated VTE.
Meaning: The results of this study suggest that ambulatory patients with COVID-19, either vaccinated or not, present a clinically relevant increased risk of incident VTE during the acute phase, with the risk pronounced by factors of older age, male sex, obesity, incomplete vaccination, and factor V Leiden thrombophilia.
Abstract
Importance: The risk of venous thromboembolism (VTE) in ambulatory COVID-19 is controversial. In addition, the association of vaccination with COVID-19-related VTE and relevant clinical and genetic risk factors remain to be elucidated.
Objective: To quantify the association between ambulatory COVID-19 and short-term risk of VTE, study the potential protective role of vaccination, and investigate clinical and genetic risk factors for post-COVID-19 VTE. Design, Setting, and Participants: This population-based cohort study of patients with COVID-19 from UK Biobank included participants with SARS-CoV-2 infection that was confirmed by a positive polymerase chain test reaction result between March 1, 2020, and September 3, 2021, who were then propensity score matched to COVID-19-naive people during the same period. Participants with a history of VTE who used antithrombotic drugs (1 year before index dates) or tested positive in hospital were excluded.
Exposures: First infection with SARS-CoV-2, age, sex, ethnicity, socioeconomic status, obesity, vaccination status, and inherited thrombophilia.
Main Outcomes and Measures: The primary outcome was a composite VTE, including deep vein thrombosis or pulmonary embolism, which occurred 30 days after the infection. Hazard ratios (HRs) with 95% CIs were calculated using cause-specific Cox models.
Results: In 18 818 outpatients with COVID-19 (10 580 women [56.2%]; mean [SD] age, 64.3 [8.0] years) and 93 179 matched uninfected participants (52 177 women [56.0%]; mean [SD] age, 64.3 [7.9] years), the infection was associated with an increased risk of VTE in 30 days (incidence rate of 50.99 and 2.37 per 1000 person-years for infected and uninfected people, respectively; HR, 21.42; 95% CI, 12.63-36.31). However, risk was substantially attenuated among the fully vaccinated (HR, 5.95; 95% CI, 1.82-19.5; interaction P = .02). In patients with COVID-19, older age, male sex, and obesity were independently associated with higher risk, with adjusted HRs of 1.87 (95% CI, 1.50-2.33) per 10 years, 1.69 (95% CI, 1.30-2.19), and 1.83 (95% CI, 1.28-2.61), respectively. Further, inherited thrombophilia was associated with an HR of 2.05 (95% CI, 1.15-3.66) for post-COVID-19 VTE.
Conclusions and Relevance: In this population-based cohort study of patients with COVID-19, ambulatory COVID-19 was associated with a substantially increased risk of incident VTE, but this risk was greatly reduced in fully vaccinated people with breakthrough infection. Older age, male sex, and obesity were clinical risk factors for post-COVID-19 VTE; factor V Leiden thrombophilia was additionally associated with double the risk, comparable with the risk of 10-year aging. These findings may reinforce the need for vaccination, inform VTE risk stratification, and call for targeted VTE prophylaxis strategies for unvaccinated outpatients with COVID-19
(13). Anand R. Habib, et al. Statins for primary cardiovascular disease prevention, time to curb our enthusiasm. JAMA Intern Med. 2022;182(10):1021-4.
In the US, more than 126 million adults have been diagnosed with cardiovascular disease (CVD).Reducing the morbidity and mortality associated with CVD is a public health imperative. Accordingly, considerable resources and effort have been invested in determining not only how to effectively treat symptomatic coronary artery disease or ischemic stroke, but also on the prevention of clinical CVD. Although elevated low-density lipoprotein (LDL) is associated with higher rates of CVD, there is uncertainty regarding the net benefit to risk ratio of using statins to reduce LDL among persons without CVD (primary prevention). This contrasts with the established role of LDL reduction for persons with established CVD (secondary prevention).
The US Preventive Services Task Force (USPSTF) has updated its 2016 recommendations on the use of statins for the primary prevention of clinical CVD. Two of us (M.H.K. and R.F.R.) wrote about the 2016 recommendations, and in this editorial, we update our comments for the 2022 recommendations.
The 2022 recommendations seem largely unchanged from the 2016 recommendations. For individuals aged 40 to 75 years without clinical CVD, with LDL lower than 190 mg/dL, and without known familial hypercholesterolemia, the USPSTF makes 3 recommendations. First, the Task Force concludes with moderate certainty that those with an estimated 10-year CVD event risk of 10% or higher based on the American College of Cardiology/American Heart Association pooled cohort equations (PCEs) and at least 1 risk factor-dyslipidemia (LDL of >130 mg/dL or high-density lipoprotein of <40 mg/dL), diabetes, hypertension, and/or smoking-are likely to derive moderate benefit (B recommendation) from initiation of a moderate-intensity statin (lowers LDL by 30%-49% on average). Second, the Task Force concludes with moderate certainty that those with an estimated 10-year CVD event risk of 7.5% to less than 10% and at least 1 risk factor are likely to experience small net benefit (C recommendation) from initiation of a moderate-intensity statin, and thus clinicians should engage patients in shared decision-making. Third, the Task Force concludes that there is insufficient evidence (I statement) to fully assess the net harms and benefits of initiating statins in adults 76 years and older, regardless of estimated 10-year CVD event risk or presence of risk factors.
The details of the updated recommendations merit further consideration. The systematic review6 that accompanies the 2022 USPSTF recommendations examined 22 randomized clinical trials (RCTs; n = 90 624 participants) that compared statin therapy vs placebo or no statin, with a mean follow-up duration of 3 years. The systematic review examined the clinical end points of all-cause mortality, CVD mortality, fatal/nonfatal myocardial infarction, and fatal/nonfatal stroke among those with a mean age of 52 to 66 years, except for 1 trial that only enrolled older individuals between 70 and 82 years of age
Clinical End Points Analyzed in the Systematic Reviews for the USPSTF
The updated evidence synthesis6 found that statins yielded a slightly smaller, but still statistically significant, reduction in all-cause mortality (pooled relative risk, 0.92; 95% CI, 0.87-0.98), as well as for myocardial infarction and stroke . The USPSTF recommendations should be considered in the context of a meta-analysis, published in 2010, which included only trials that enrolled patients receiving high-risk primary prevention; this study showed no benefit on all-cause mortality with statins. The benefit for CVD mortality was not statistically significant (pooled relative risk, 0.91; 95% CI, 0.81-1.02). Notably, there was no significant statistical heterogeneity (I2 = 0) across the 12 trials (n = 75 138) examined. The null result was robust in sensitivity analyses that excluded trials that stopped early, trials that included patients receiving secondary prevention, good-quality trials, trials with at least 3 years of follow-up, and trials with participants with a median baseline LDL lower than 160 mg/dL. The lack of benefit on CVD mortality found in the 2022 evidence review6 and the lack of benefit on all-cause mortality in the purely high-risk primary prevention meta-analysis8 call into question the reliability of the all-cause mortality benefit reported in the systematic review that accompanies the 2022 USPSTF recommendations. Because 19 of the 22 included trials were industry sponsored, potential bias in the randomization processes, as well as the potential for exaggeration of net benefits, are of greater concern than for trials whose sponsors were disinterested with regard to the outcomes.
In contrast with its 2016 recommendations, the USPSTF no longer recommends use of low-intensity statins in certain situations. This change is driven by the fact that 17 of the 22 trials had fixed-dose statin groups, including 12 which used moderate-intensity statins. We are unaware of any RCTs that directly compare either different statin intensities in terms of clinical CVD outcomes or fixed-dose vs titrate-to-LDL goal approaches. While it is understandable that the Task Force was limited by lack of data on dosing, this change is unfortunate for patients because the frequency of adverse effects increases as the statin dose increases.
Finally, the 2022 recommendations no longer note the “uncertainty in individual risk prediction” in contrast with the 2016 recommendations, which more clearly acknowledged that cardiac risk prediction is not an exact science. Refining the accuracy of prediction of risk for individuals remains a major issue for primary prevention. It is disappointing that there was no sex-specific analysis in the evidence review, as women have lower cardiovascular risk than men at all ages until age 75 years and are more likely to experience adverse drug effects than men. Thus, the risk-benefit profile for statins is less favorable for women than men, which is neither discussed nor recognized in the current USPSTF recommendations.
Many of the caveats and concerns noted in the Editorial accompanying the 2016 USPSTF recommendations are still present with the 2022 recommendations. These include the heterogeneous inclusion criteria across studies and the inclusion of participants not receiving primary prevention who either had symptoms consistent with clinical CVD or known coronary artery disease equivalents, such as carotid artery atherosclerosis. As noted previously, a meta-analysis of statins for patients receiving high-risk primary prevention found no benefit on all-cause mortality.8 Additionally, as the data for many trials of statin therapy remain unavailable, the USPSTF does not have access to participant-level data. The trials for which there is no data transparency are those with data housed at the Cholesterol Treatment Trialists’ (CTT) Collaborative at Oxford University. The CTT does not allow academic researchers and others access to the data from 24 trials comparing statins with a control group, starting in 1995. The CTT states that it is just the repository of data and cannot allow others access because the data are owned and controlled by the industry sponsors of the trials. In 2022, there is no reason for participant-level data from these trials to remain unavailable for independent analysis.
At present, there are further reasons to curb our enthusiasm about the use of statins for primary prevention of CVD. There is a difference between statistically significant and clinically meaningful benefit. The purported benefits of statins in terms of relative risk reduction are fairly constant across baseline lipid levels and cardiovascular risk score categories for primary prevention. Therefore, the absolute benefit for those in lower-risk categories is likely small given that their baseline absolute risk is low, while the chance of adverse effects is constant across risk categories.
Use of the PCE to risk-stratify individuals is problematic. The cut points of 5%, 7.5%, 10%, and 20% are arbitrary given the linear “continuum of risk” without a threshold effect3 that exists within the relationship between CVD risk and CVD occurrence. Additionally, the PCE itself is an imperfect tool to assess baseline 10-year absolute risk. The PCE was derived and validated in studies that enrolled individuals (mostly White males) between 1968 and 1990. Thus, the PCE does not reflect the recent decreases in rates of CVD that have accrued owing to population-wide health improvements from reduced rates of smoking, shifts in dietary patterns and exercise, and blood pressure control. In a multiethnic prospective cohort (n = 4227) from 2000 to 2002, the PCE overestimated CVD events by 86% in men and 67% in women. When applied to 6 cohorts from 1971 to 2014 (n = 26 689), the PCE overestimated 10-year risk of CVD by an average of 20% across risk groups, which would have down-staged 11.7 million adults from a 10-year CVD risk of greater than 10% to less than 10%, and 11.8 million adults from a 10-year CVD risk of greater than 7.5% to less than 7.5%. Furthermore, the PCE propagates the unavoidable variability and uncertainty in the clinical inputs (eg, total cholesterol, high-density lipoprotein, systolic blood pressure); a 10% higher or lower variation in these inputs would result in up to a 24% change in the risk categorization of individuals. Such issues with discrimination and calibration present challenges in applying the USPSTF guidelines to individuals in informing discussions about their potential for clinical benefits from statin use.
The USPSTF concluded that, among 19 RCTs and 3 observational studies examined (a different data set than the data set for the review of benefits, which was 22 RCTs),6statins did not have any statistically significant harms, namely in terms of myalgias, incident diabetes, liver enzyme elevations, cancer, kidney effects, cognitive harms, or cataracts. However, in clinical practice, adverse events are commonly reported with use of statins. For example, in observational data, statin-associated muscle symptoms affect up to 1 in 10 individuals. Even if, as has been argued, statin-associated muscle symptoms are at least partly due to the nocebo effect, the extent to which muscle symptoms lead to either dose-reduction or discontinuation of statins (usually with subsequent cessation of these symptoms) should not be discounted. Although the USPSTF analysis6 did not find a statistically significant increase in incident diabetes, a prior meta-analysis of 13 trials (n = 91 140) found that 1 extra case of diabetes per 255 patients over 4 years of statin treatment could be attributed to statin use. Furthermore, individuals with other risk factors for glycemic intolerance and people with preexisting diabetes are likely to be at increased risk of progression to diabetes and worsened glycemic control, respectively. When assessing the potential harms of statins, it is prudent to keep in mind that although the purported benefits of statins will accrue to a few patients in the future, everyone prescribed a moderate-intensity statin is at risk immediately for the harms. Consideration of adverse effects is especially important for a primary prevention drug, which is prescribed to healthy people who feel perfectly well.
The practice of medicine is an art as well as a science. As the USPSTF and other professional societies, including the American College of Cardiology/American Heart Association24 and the European Society of Cardiology/European Atherosclerosis Society have all emphasized, shared decision-making of the anticipated benefits, harms, and uncertainties in predicting CVD are essential in determining whether to initiate a medication that a patient may possibly take for the remainder of their life. Although there are patient-facing decision-support aids for statins, they are underused. These aids would be more useful if quality-of-life data were collected in statin trials and could be added to decision-making tools. In addition, consideration should be given to deprescribing statins for adults 76 years or older, other older individuals unlikely to derive benefit from statins for primary prevention, and individuals who are at risk of polypharmacy because of medications taken for other conditions.
In the US, about $25 billion is spent annually on statins. Cardiovascular disease incidence and mortality are the upshot of myriad social determinants. Although statins lower LDL cholesterol in individuals, investments at the community level to construct a more salubrious environment that enables healthy eating and promotes physical activity are likely to have more widespread multiplicative and pleiotropic effects on the biological and psychosocial risks of CVD, as well as on improving quality of life. The 2022 USPSTF recommendations are an opportunity to pause and refocus efforts to meaningfully improve CVD outcomes for all, rather than extol the marginal, likely small, and uncertain absolute benefits of statins for the few in primary CVD prevention.
(14). Michelle Roberts. Stem cell patch surgery to mend spina bifida in the womb. https://www.bbc.com/news/health-63155654
US doctors say they have successfully carried out surgery on babies in the womb to repair harmful spine defects using a special, therapeutic stem cell patch method.
Experts hope the pioneering research at UC Davis Health could help others with spina bifida – when the spinal cord and spine do not develop properly.
Three of the babies in the trial have now been born.
The team will monitor them for at least six years.
Without treatment, spina bifida can sometimes lead to a range of lifelong issues, including problems with mobility because of nerve damage. In extreme cases, the spinal canal remains open and exposed. If the defect is not closed to protect it shortly before or after birth, it can cause total paralysis of the legs.
Surgeons have already used keyhole surgery on babies in the womb to mend the gap. Now the US team have gone a step further, fitting a graft or implant to bridge the repair.
It is a patch that contains immature cells, called stem cells, that can grow with the baby.
The researchers say they have already had very promising results in animals with this technique. They have tried it in baby sheep and a pair of English bulldog puppies – Darla and Spanky – to refine the process.
Baby Robbie is one of the first humans to have the treatment.
Her mum, Emily, says it was a lifeline that they could not refuse.
“We didn’t know about spina bifida until the diagnosis. We are so thankful that we got to be a part of this. We are giving our daughter the very best chance at a bright future,” she said.
Their surgeon, Diana Farmer, says the operation went off without a hitch: “Mother and foetus did great!”
Emily recalls the day Robbie was born more than a year ago.
“One of my first fears was that I wouldn’t be able to see her, but they brought her over to me. I got to see her toes wiggle for the first time. It was so reassuring and a little bit out of this world.”
The UC Davis team plan to treat about 35 babies as part of their trial. More studies and follow-up are needed to assess how well the treatment works.
Robbie and the other babies will have check-ups to see their progress with skills such as walking and potty training.
Prof Paolo De Coppi is a paediatric surgeon at London’s Great Ormond Street Hospital in the UK. He has been doing surgery on babies in the womb for spina bifida and says using stem cell patches might improve outcomes even further. “That’s the hope, but we need to wait and see,” he told the BBC.
It is not known what causes spina bifida but a number of things can increase the risk of a baby developing the condition.
Not having enough folic acid (vitamin B9) during pregnancy is one of the most important ones.
Prof Neena Modi, an expert in neonatal medicine at Imperial College London in the UK, stressed the importance of women taking supplements around conception and in pregnancy – a cheap and easy intervention that can help prevent cases of spina bifida
(15). Chunrong Tao, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022; 387:1361-72.
Background
Data from trials investigating the effects and risks of endovascular thrombectomy for the treatment of stroke due to basilar-artery occlusion are limited.
Methods
We conducted a multicenter, prospective, randomized, controlled trial of endovascular thrombectomy for basilar-artery occlusion at 36 centers in China. Patients were assigned, in a 2:1 ratio, within 12 hours after the estimated time of basilar-artery occlusion to receive endovascular thrombectomy or best medical care (control). The primary outcome was good functional status, defined as a score of 0 to 3 on the modified Rankin scale (range, 0 [no symptoms] to 6 [death]), at 90 days. Secondary outcomes included a modified Rankin scale score of 0 to 2, distribution across the modified Rankin scale score categories, and quality of life. Safety outcomes included symptomatic intracranial hemorrhage at 24 to 72 hours, 90-day mortality, and procedural complications.
Results
Of the 507 patients who underwent screening, 340 were in the intention-to-treat population, with 226 assigned to the thrombectomy group and 114 to the control group. Intravenous thrombolysis was used in 31% of the patients in the thrombectomy group and in 34% of those in the control group. Good functional status at 90 days occurred in 104 patients (46%) in the thrombectomy group and in 26 (23%) in the control group (adjusted rate ratio, 2.06; 95% confidence interval [CI], 1.46 to 2.91, P<0.001). Symptomatic intracranial hemorrhage occurred in 12 patients (5%) in the thrombectomy group and in none in the control group. Results for the secondary clinical and imaging outcomes were generally in the same direction as those for the primary outcome. Mortality at 90 days was 37% in the thrombectomy group and 55% in the control group (adjusted risk ratio, 0.66; 95% CI, 0.52 to 0.82). Procedural complications occurred in 14% of the patients in the thrombectomy group, including one death due to arterial perforation.
Conclusions
In a trial involving Chinese patients with basilar-artery occlusion, approximately one third of whom received intravenous thrombolysis, endovascular thrombectomy within 12 hours after stroke onset led to better functional outcomes at 90 days than best medical care but was associated with procedural complications and intracerebral hemorrhage.
(16). R MacGregor, et al. Investigating abnormal uterine bleeding in reproductive aged women. BMJ 2022;378:e070906
What you need to know
Abnormal uterine bleeding (AUB) is common, and the cause must be understood in order to direct management
AUB in the perimenopause is a risk factor for endometrial hyperplasia or malignancy, and investigation is warranted
Iron deficiency and iron deficiency anaemia are extremely common in women with AUB and often easily remedied
Abnormal uterine bleeding (AUB) is a common presentation in primary care. Estimates vary, but the prevalence among non-pregnant women of reproductive age globally is thought to be between 20% and 35%.123 This article refers to women, but the concepts apply to all people who menstruate. AUB affects women of all ages and backgrounds, with women from ethnic minority backgrounds and those living in deprivation the least likely to seek or receive treatment.3 In the past 10 years, systems for the nomenclature and classification of AUB have been established, with the International Federation of Obstetrics and Gynaecology (FIGO) publishing guidance in 2011 and updated in 2018 to guide patient care and management of those with AUB in the reproductive years.24 The UK’s National Institute for Health and Care Excellence (NICE) also published updated guidance for heavy menstrual bleeding in 2018.
Definitions
Abnormal uterine bleeding is the term used to encompass the symptoms of heavy menstrual bleeding and intermenstrual bleeding, and describes any bleeding from the uterus that is abnormal in flow volume, regularity, frequency, or duration.4 AUB is a symptom not a diagnosis
(17). Ghadeer K. Dawwas, et al. Apixaban versus rivaroxaban in patients with atrial fibrillation and valvular heart disease, a population-based study. Ann Int Med. 2022
Background
Although apixaban and rivaroxaban are commonly used in patients with atrial fibrillation (AF) and valvular heart disease (VHD), there is limited evidence comparing the 2 drugs in these patients.
Objective
To emulate a target trial of effectiveness and safety of apixaban and rivaroxaban in patients with AF and VHD.
Design
New-user, active comparator, cohort study design.
Setting
Commercial health insurance database from 1 January 2013 to 31 December 2020.
Patients
New users of apixaban or rivaroxaban who had a diagnosis of AF and VHD before initiation of anticoagulant therapy.
Measurements
The primary effectiveness outcome was a composite of ischemic stroke or systemic embolism. The primary safety outcome was a composite of gastrointestinal or intracranial bleeding. Cox proportional hazards regression with a robust variance estimator was used to estimate hazard ratios (HRs) and 95% CIs.
Results
When compared with rivaroxaban in a propensity score–matched cohort of 19 894 patients (9947 receiving each drug), apixaban was associated with a lower rate of ischemic stroke or systemic embolism (HR, 0.57 [95% CI, 0.40 to 0.80]) and bleeding (HR, 0.51 [CI, 0.41 to 0.62]). The absolute reduction in the probability of stroke or systemic embolism with apixaban compared with rivaroxaban was 0.0026 within 6 months and 0.011 within 1 year of treatment initiation. The absolute reduction in the probability of bleeding events with apixaban compared with rivaroxaban was 0.012 within 6 months and 0.019 within 1 year of treatment initiation.
Limitation
Short follow-up time and inability to ascertain some types of VHD.
Conclusion
In this study of patients with AF and VHD, patients receiving apixaban had a lower risk for ischemic stroke or systemic embolism and for bleeding when compared with those receiving rivaroxaban.
Primary Funding Source: National Institutes of Health
(18). David Rosmarin, et al. Two Phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-55.
Background
Vitiligo is a chronic autoimmune disease that causes skin depigmentation. A cream formulation of ruxolitinib (an inhibitor of Janus kinase 1 and 2) resulted in repigmentation in a phase 2 trial involving adults with vitiligo.
Methods
We conducted two phase 3, double-blind, vehicle-controlled trials (Topical Ruxolitinib Evaluation in Vitiligo Study 1 [TRuE-V1] and 2 [TRuE-V2]) in North America and Europe that involved patients 12 years of age or older who had nonsegmental vitiligo with depigmentation covering 10% or less of total body-surface area. Patients were randomly assigned in a 2:1 ratio to apply 1.5% ruxolitinib cream or vehicle control twice daily for 24 weeks to all vitiligo areas on the face and body, after which all patients could apply 1.5% ruxolitinib cream through week 52. The primary end point was a decrease (improvement) of at least 75% from baseline in the facial Vitiligo Area Scoring Index (F-VASI; range, 0 to 3, with higher scores indicating a greater area of facial depigmentation), or F-VASI75 response, at week 24. There were five key secondary end points, including improved responses on the Vitiligo Noticeability Scale.
Results
A total of 674 patients were enrolled, 330 in TRuE-V1 and 344 in TRuE-V2. In TRuE-V1, the percentage of patients with an F-VASI75 response at week 24 was 29.8% in the ruxolitinib-cream group and 7.4% in the vehicle group (relative risk, 4.0; 95% confidence interval [CI], 1.9 to 8.4; P<0.001). In TRuE-V2, the percentages were 30.9% and 11.4%, respectively (relative risk, 2.7; 95% CI, 1.5 to 4.9; P<0.001). The results for key secondary end points showed superiority of ruxolitinib cream over vehicle control. Among patients who applied ruxolitinib cream throughout 52 weeks, adverse events occurred in 54.8% in TRuE-V1 and 62.3% in TRuE-V2; the most common adverse events were application-site acne (6.3% and 6.6%, respectively), nasopharyngitis (5.4% and 6.1%), and application-site pruritus (5.4% and 5.3%).
Conclusions
In two phase 3 trials, application of ruxolitinib cream resulted in greater repigmentation of vitiligo lesions than vehicle control through 52 weeks, but it was associated with acne and pruritus at the application site. Larger and longer trials are required to determine the effect and safety of ruxolitinib cream in patients with vitiligo.
(19). Henrik Schmidt, et al. Oxygen targets in comatose survivors of cardiac arrest. N Engl J Med 2022; 387:1467-1476.
Background
The appropriate oxygenation target for mechanical ventilation in comatose survivors of out-of-hospital cardiac arrest is unknown.
Methods
In this randomized trial with a 2-by-2 factorial design, we randomly assigned comatose adults with out-of-hospital cardiac arrest in a 1:1 ratio to either a restrictive oxygen target of a partial pressure of arterial oxygen (Pao2) of 9 to 10 kPa (68 to 75 mm Hg) or a liberal oxygen target of a Pao2 of 13 to 14 kPa (98 to 105 mm Hg); patients were also assigned to one of two blood-pressure targets (reported separately). The primary outcome was a composite of death from any cause or hospital discharge with severe disability or coma (Cerebral Performance Category [CPC] of 3 or 4; categories range from 1 to 5, with higher values indicating more severe disability), whichever occurred first within 90 days after randomization. Secondary outcomes were neuron-specific enolase levels at 48 hours, death from any cause, the score on the Montreal Cognitive Assessment (ranging from 0 to 30, with higher scores indicating better cognitive ability), the score on the modified Rankin scale (ranging from 0 to 6, with higher scores indicating greater disability), and the CPC at 90 days.
Results
A total of 789 patients underwent randomization. A primary-outcome event occurred in 126 of 394 patients (32.0%) in the restrictive-target group and in 134 of 395 patients (33.9%) in the liberal-target group (hazard ratio, 0.95; 95% confidence interval, 0.75 to 1.21; P=0.69). At 90 days, death had occurred in 113 patients (28.7%) in the restrictive-target group and in 123 (31.1%) in the liberal-target group. On the CPC, the median category was 1 in the two groups; on the modified Rankin scale, the median score was 2 in the restrictive-target group and 1 in the liberal-target group; and on the Montreal Cognitive Assessment, the median score was 27 in the two groups. At 48 hours, the median neuron-specific enolase level was 17 μg per liter in the restrictive-target group and 18 μg per liter in the liberal-target group. The incidence of adverse events was similar in the two groups.
Conclusions
Targeting of a restrictive or liberal oxygenation strategy in comatose patients after resuscitation for cardiac arrest resulted in a similar incidence of death or severe disability or coma.
(20). Jesper Kjaergaard, et al. Blood-pressure targets in comatose survivors of cardiac arrest. N Engl J Med 2022;387:1456-66
Background
Evidence to support the choice of blood-pressure targets for the treatment of comatose survivors of out-of-hospital cardiac arrest who are receiving intensive care is limited.
Methods
In a double-blind, randomized trial with a 2-by-2 factorial design, we evaluated a mean arterial blood-pressure target of 63 mm Hg as compared with 77 mm Hg in comatose adults who had been resuscitated after an out-of-hospital cardiac arrest of presumed cardiac cause; patients were also assigned to one of two oxygen targets (reported separately). The primary outcome was a composite of death from any cause or hospital discharge with a Cerebral Performance Category (CPC) of 3 or 4 within 90 days (range, 0 to 5, with higher categories indicating more severe disability; a category of 3 or 4 indicates severe disability or coma). Secondary outcomes included neuron-specific enolase levels at 48 hours, death from any cause, scores on the Montreal Cognitive Assessment (range, 0 to 30, with higher scores indicating better cognitive ability) and the modified Rankin scale (range, 0 to 6, with higher scores indicating greater disability) at 3 months, and the CPC at 3 months.
Results
A total of 789 patients were included in the analysis (393 in the high-target group and 396 in the low-target group). A primary-outcome event occurred in 133 patients (34%) in the high-target group and in 127 patients (32%) in the low-target group (hazard ratio, 1.08; 95% confidence interval [CI], 0.84 to 1.37; P=0.56). At 90 days, 122 patients (31%) in the high-target group and 114 patients (29%) in the low-target group had died (hazard ratio, 1.13; 95% CI, 0.88 to 1.46). The median CPC was 1 (interquartile range, 1 to 5) in both the high-target group and the low-target group; the corresponding median modified Rankin scale scores were 1 (interquartile range, 0 to 6) and 1 (interquartile range, 0 to 6), and the corresponding median Montreal Cognitive Assessment scores were 27 (interquartile range, 24 to 29) and 26 (interquartile range, 24 to 29). The median neuron-specific enolase level at 48 hours was also similar in the two groups. The percentages of patients with adverse events did not differ significantly between the groups.
Conclusions
Targeting a mean arterial blood pressure of 77 mm Hg or 63 mm Hg in patients who had been resuscitated from cardiac arrest did not result in significantly different percentages of patients dying or having severe disability or coma.
