Journal Scan
A review of 13 recent papers of immediate clinical significance, harvested from major international journals
(1) Wei-Kai H, Wen-Hung C. Drug Reaction with Eosinophilia and Systemic Symptoms. N Engl J Med. 2022;387:167.
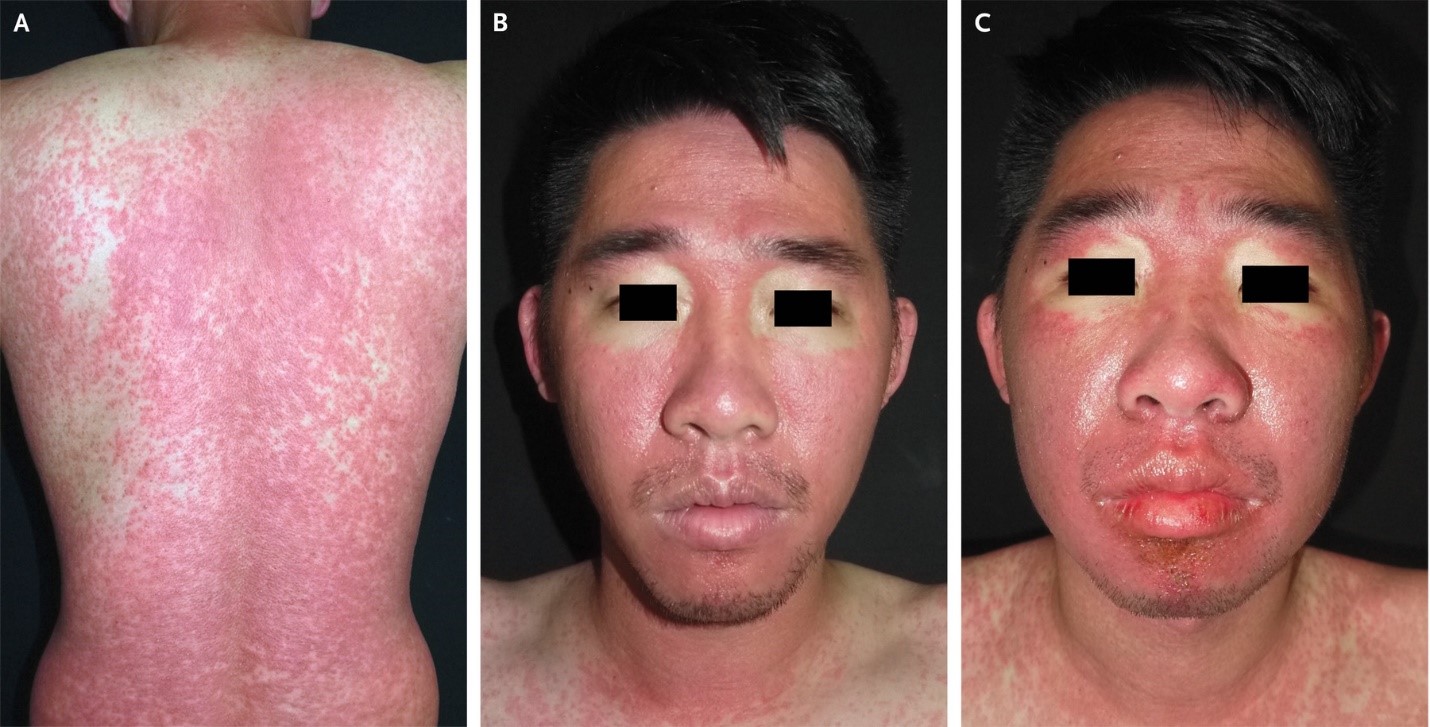
A 30-year-old man was admitted to the hospital with a 2-week history of rash and fever. Four weeks before presentation, he had taken trimethoprim-sulfamethoxazole for the treatment of folliculitis. His body temperature was 39.1°C. Physical examination revealed a morbilliform rash across the trunk, arms, and legs (Panel A), submandibular lymphadenopathy, and facial erythema with periorbital sparing (Panel B). Four days after admission, facial edema developed (Panel C). Peak laboratory values during the hospital stay included an absolute eosinophil count of 3028 per cubic millimeter (reference range, 40 to 350), an alanine aminotransferase level of 989 U per liter (reference value, <36), and an aspartate aminotransferase level of 162 U per liter (reference value, <34). The serum creatinine level was normal, as were a urinalysis, chest radiograph, antinuclear antibody test, and tests for viral hepatitis and Mycoplasma pneumoniae infection. Skin biopsy revealed interface dermatitis with eosinophilic and lymphocytic infiltration. The European Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) score was 7, which indicated a definitive diagnosis of drug reaction with eosinophilia and systemic symptoms (DRESS) related to treatment with trimethoprim-sulfamethoxazole. (RegiSCAR scores range from -4 to 9, with higher scores indicating a more definite diagnosis; a score of >5 indicates a definitive diagnosis.) Treatment with systemic glucocorticoids and cyclosporine was initiated, and the patient was advised to avoid sulfonamide-containing antimicrobial agents. At 1 month of follow-up, his symptoms had abated.
(2). Jacqui Wise. Covid-19: Infection raises risk of diabetes and heart disease diagnoses in following weeks, study finds. BMJ 2022;378:o1838.
Patients who contract covid-19 are at increased risk of being diagnosed with cardiovascular disorders and diabetes in the three months following infection, although the risk then declines back to baseline levels, a large UK study has found.
Researchers from King’s College London say patients recovering from covid-19 should be advised to consider measures to reduce diabetes risk including adopting a healthy diet and taking exercise.
The GP medical records from more than 428 650 covid-19 patients were matched with the same number of controls and followed up to January 2022. All patients with pre-existing diabetes or cardiovascular disease were excluded from the study, published in the open access journal PLOS Medicine.
According to the analysis, diabetes mellitus diagnoses were increased by 81% in acute covid-19 and remained elevated by 27% from 4 to 12 weeks after infection (adjusted rate ratio 1.81, 95% confidence interval 1.51 to 2.19).
Acute covid-19 was associated with a sixfold increase in cardiovascular diagnoses overall (adjusted rate ratio 5.82, 95% CI 4.82 to 7.03). This included an 11-fold increase in pulmonary embolism, a sixfold increase in atrial arrhythmias, and a fivefold increase in venous thromboses.
The risk of a new heart disease diagnosis began to decline five weeks after infection and returned to baseline levels or below from 12 weeks to one year.
Lead study author Emma Rezel-Potts said, “While it is in the first four weeks that covid-19 patients are most at risk of these outcomes, the risk of diabetes mellitus remains increased for at least 12 weeks. Clinical and public health interventions focusing on reducing diabetes risk among those recovering from covid-19 over the longer term may be beneficial.”
The researchers said that people without pre-existing cardiovascular disease or diabetes who become infected with covid-19 do not appear to have a long-term increase in incidence of these conditions.
The observational nature of the study means the researchers can’t say whether the short-term increase in risk is directly because of covid infection or if undiagnosed cardiovascular disease and diabetes may be more prevalent among covid-19 cases. Alternatively, covid-19 infection may have aggravated or altered the natural history of pre-existing disease.
Another possibility is that patients recovering from covid-19 had higher rates of GP consultations and this increased medical surveillance could be associated with more frequent opportunities for diabetes to be diagnosed. However, illness from diabetes could itself lead to increased consultations.
Although the researchers matched the controls for age and sex they did not have sufficient data for alcohol use, diet, physical activity, and lipid parameters. They also had no data on deprivation, although the controls were matched for family practice. Patients diagnosed with covid-19 were generally less healthy than controls and this might, in part, account for differences in cardiometabolic outcomes.
Commenting on the study, Kevin McConway, emeritus professor of applied statistics at the Open University, said the study was good in many ways. But he said, “Though the cases and the controls were matched in terms of some factors, it wouldn’t have been possible to match them on everything that is potentially relevant. The covid cases, for example, had a higher tendency to be overweight than the controls, and had on average more pre-existing health conditions. So maybe the observed difference in risk is because of, in part or entirely, these other differences between the groups, rather than the covid itself.”
(3). Giordano D, Pernice C. Retropharyngeal Abscess. N Engl J Med. 2022;387:260.
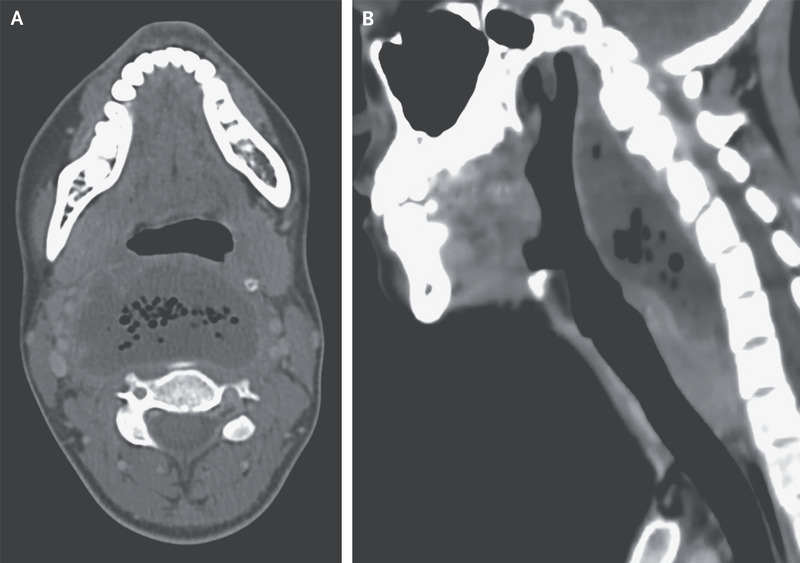
A 26-year-old woman with a history of recurrent tonsillitis presented to the emergency department with a 2-week history of progressive sore throat and a 7-day history of pain when swallowing, shortness of breath, and fever. She reported no recent sexual contact or throat trauma. On physical examination, there was marked swelling of the posterior oropharynx. Laboratory evaluations showed a white-cell count of 19,450 cells per cubic millimeter (reference range, 4000 to 10,000). Computed tomography of the neck showed a collection of fluid posterior to the pharynx measuring 6.2 by 3.4 cm, with air bubbles and a contrast-enhancing rim (Panel A), findings consistent with a retropharyngeal abscess. The fluid collection measured 10 cm on the sagittal axis (Panel B), but there was no mediastinal involvement and no thrombosis of the internal jugular vein. Urgent surgical drainage was performed, and treatment with broad-spectrum antimicrobial agents was initiated intravenously. Fluid cultures grew Fusobacterium necrophorum. Retropharyngeal abscesses can be life-threatening owing to the risks of severe sepsis, airway obstruction, and contiguous spread to the mediastinum. If there is clinical suspicion of retropharyngeal abscess, cross-sectional imaging should be performed. By postoperative day 7, the patient’s symptoms had abated, and she was discharged with oral antimicrobial agents.
(4). Boyce M, Flower C. Tripe Palms. 2022.
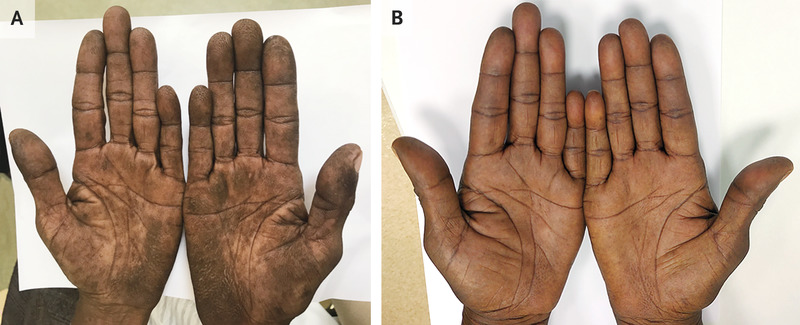
A 59-year-old woman with a 35-pack-year history of smoking tobacco presented to the clinic with a 5-month history of skin darkening on the palms. She reported no other symptoms. Physical examination showed velvety thickening of the skin on her palms and fingers, with accentuated fingerprint ridges, a finding known as tripe palms, or acanthosis palmaris (Panel A). There was also shortening of the small finger on both hands, a finding that was thought to be a normal variation. Neither clubbing nor acanthosis nigricans was present. Owing to concern for underlying cancer, computed tomography of the chest was performed, and a mass was observed in the upper lobe of the right lung. A biopsy specimen of the mass was obtained and revealed undifferentiated carcinoma. A right upper lobectomy was then performed, and moderately differentiated, invasive lung adenocarcinoma with metastases to regional lymph nodes was observed. Testing of the tumor for mutations was not available owing to resource limitations. Tripe palms – named for their resemblance to the gastric lining of ruminant animals – are a type of paraneoplastic dermatopathy seen most frequently in patients with adenocarcinoma of the lungs or the gastrointestinal tract. Cancer treatment may resolve this finding, as it did in this patient. After completion of a 5-month course of systemic chemotherapy, the skin changes had abated (Panel B).
(5). Christopher McChesney, et al. Do not routinely test for vitamin D. BMJ 2022;378:e070270.
What you need to know
Routinely testing vitamin D levels in asymptomatic individuals is not recommended, based on a lack of evidence for benefit
Shared decision making and conversations with patients can help explore the risks and benefits of unnecessary testing
Physician education, audit, feedback of physicians’ ordering practices, and system-wide changes in ordering and remuneration are effective strategies to reduce unnecessary vitamin D testing
Routine vitamin D testing has been increasing owing to patient demand, attention in mass media, correlational studies connecting vitamin D to various health concerns, and physicians promoting its use.12345678910 Recently, possible associations between vitamin D deficiency and severe covid-19 received scientific attention, fuelling renewed media attention and a rapid guideline from the National Institute for Health and Care Excellence (NICE) on appropriate indications for supplementation.111213141516
Box 1 presents a list of clinical conditions where testing vitamin D may be appropriate, but vitamin D tests are frequently requested without any of these clinical indications. Studies from the UK, US, Canada, and Australia suggest that 25% to 75% of vitamin D testing may be unnecessary.92223242526 This can be potentially harmful to patients by leading to additional testing, and wastes valuable healthcare resources.27 In this article we offer an overview of evidence and guideline recommendations on routine vitamin D testing, (also known as vitamin D screening) and offer practical suggestions for reducing unnecessary testing.
Box 1
Commonly cited indications where vitamin D testing is appropriate1718192021
Rickets
Osteomalacia
Osteoporosis
Hyperparathyroidism
Malabsorption syndromes
Medications affecting absorption or metabolism of vitamin D (antifungals, HIV antiretroviral therapy, anticonvulsants, etc)
Chronic kidney disease
Hypophosphatemia and hypo/hypercalcemia
Deeply pigmented skin
Isolated elevation of alkaline phosphatase
RETURN TO TEXT
Evidence for change
Several international guidelines recommend against routinely testing or screening for vitamin D in people with no clinical symptoms or risk of deficiency…
(6). Limb M. Concurrent surgery across two parallel operating theatres carries risks, say researchers. BMJ 2022;378:o1877
Researchers have warned of the risks of having a single surgeon work across two parallel operating theatres and cast doubt on the method as a means of treating more patients to cut NHS waiting lists.
They say there is no evidence that “overlapping surgery” delivers increased productivity compared with two surgeons focused on their own lists, and warn that there are proven “small but very real risks” to patient outcomes, safety, and training.
Researchers led by Jaideep Pandit, clinical director of operating theatres at Oxford University Hospitals NHS Foundation Trust, express their concerns in a paper published in Anaesthesia.
Some NHS trusts, including Guy’s and St Thomas’, in London, are trialling versions of overlapping surgery, known as high intensity theatre (HIT), to help reduce the backlog for non-emergency surgery caused by the pandemic.
Pandit told The BMJ that he could not comment on individual trusts’ initiatives and there was potential for overlapping surgery to have some positive impact in certain situations. But he said that given that a lot of infrastructure and support seemed to be going into making overlapping surgery happen, he was concerned there had been “no literature based discussion around the policy in the UK.”
“The point of our paper is to say we need to be extremely cautious before we start,” he said. “Particular caution is needed to take into account patient safety and consent and training, and what one gets out of it in terms of productivity.”
What is overlapping surgery?
Overlapping surgery involves organising surgical lists in parallel, with a single senior surgeon moving across two operating theatres. Anaesthetists induce anaesthesia and junior surgeons commence and complete the operations in both theatres.
A single senior surgeon performs the critical parts of surgery on the first patient before joining the second patient to carry out the critical parts of surgery for them.
This requires trainees of sufficient seniority to start non-critical parts of surgery as well as not being adversely affected by missing out on being present for critical parts.
Safety concerns have been raised previously. In their review, the Anaesthesia paper authors cited evidence from Canada showing overlapping surgery led to an almost doubling of risk of complications for both hip fracture and hip arthroplasty.
“The greater the overlap, the more risk there is of overlap impinging on the ‘critical portion’ of the surgery and, therefore, the greater the risk of harm,” they wrote.
According to the paper, a “disastrous attempted rollout” of overlapping surgery in the US resulted in multimillion dollar lawsuits, driven by lack of proper consent. Patients and insurers did not know the senior surgeon would not be present throughout the surgery.
Productivity claims
The authors said claims for the success of the method in raising productivity were wrong -because they focused only on the gains made by a single surgeon, maximising the time that an individual spent operating, rather than viewing total productivity across two operating theatres.
They said, “The downside of the one senior surgeon eliminating their own wasted time is that all other members of the team-across two operating theatres-increase their own downtime, needing to wait for the surgeon to move across theatres.”
The authors added, “It is inevitable that if an extra operating theatre is available to a single surgeon, then that surgeon’s number of cases will increase compared with operating from just one theatre. However, it is impossible (for mathematical reasons shown in the paper) for this to exceed the number of the same operations performed by two surgeons in separate theatres.”
Overlapping surgery could even result in fewer cases being completed than in two independent theatres, they said.
The authors said, “The real question is how productivity of one surgeon working across two overlapping operating theatres compares with two surgeons focused on their own lists.”
The researchers said overlapping surgery could be useful in the NHS in hard pressed specialties where there are not enough surgeons to staff more than one theatre, or where only one surgeon has volunteered for extra evening or weekend lists where there are anaesthetists and nursing staff available.
They said there was potential for positive impact in situations where turnover times between cases are long, operations are short (two hours or less), and where “critical portions” of surgery constitute about half of the total operation time.
The US experience showed there should be advanced agreement from all specialties and stakeholders, including patient or lay representatives, before overlapping surgery is introduced, the researchers said. “This should be coupled with a programme of education (in the background theory) and training (in the practical workflow changes required) for all staff involved,” the authors said.
Imran Ahmad, a consultant anaesthetist who leads the HIT lists at Guy’s and St Thomas’, said the trust’s model was “super efficient,” based on the experience of treating nearly 300 patients across seven specialties.
“Our complication rate is not higher than it would normally be and we mitigate the potential for risks by doing extra checks and team briefings, planning the list properly, and by carefully selecting the patients,” he told The BMJ.
References
Pandit JJ, Ramachandran SK, Pandit M. The effect of overlapping surgical scheduling on operating theatre productivity: a narrative review. Anaesthesia2022. doi:10.1111/anae.15797. pmid:35863080
(7). Byeong-Keuk K, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. The Lancet 2022;400(10349):P380-390.
Background
Drug combinations rather than increasing doses of one drug can achieve greater efficacy and lower risks. Thus, as an alternative to high-intensity statin monotherapy, moderate-intensity statin with ezetimibe combination therapy can lower LDL cholesterol concentrations effectively while reducing adverse effects. However, evidence from randomised trials to compare long-term clinical outcomes is needed.
Methods
In this randomised, open-label, non-inferiority trial, patients with atherosclerotic cardiovascular disease (ASCVD) at 26 clinical centres in South Korea were randomly assigned (1:1) to receive either moderate-intensity statin with ezetimibe combination therapy (rosuvastatin 10 mg with ezetimibe 10 mg) or high-intensity statin monotherapy (rosuvastatin 20 mg). The primary endpoint was the 3-year composite of cardiovascular death, major cardiovascular events, or non-fatal stroke, in the intention-to-treat population with a non-inferiority margin of 2.0%. This trial is registered with ClinicalTrials.gov, NCT03044665 and is complete.
Findings
Between Feb 14, 2017, and Dec 18, 2018, 3780 patients were enrolled: 1894 patients to the combination therapy group and 1886 to the high-intensity statin monotherapy group. The primary endpoint occurred in 172 patients (9.1%) in the combination therapy group and 186 patients (9.9%) in the high-intensity statin monotherapy group (absolute difference -0.78%; 90% CI -2.39 to 0.83). LDL cholesterol concentrations of less than 70 mg/dL at 1, 2, and 3 years were observed in 73%, 75%, and 72% of patients in the combination therapy group, and 55%, 60%, and 58% of patients in the high-intensity statin monotherapy group (all p<0.0001). Discontinuation or dose reduction of the study drug by intolerance was observed in 88 patients (4.8%) and 150 patients (8.2%), respectively (p<0.0001).
Interpretation
Among patients with ASCVD, moderate-intensity statin with ezetimibe combination therapy was non-inferior to high-intensity statin monotherapy for the 3-year composite outcomes with a higher proportion of patients with LDL cholesterol concentrations of less than 70 mg/dL and lower intolerance-related drug discontinuation or dose reduction
(8). Dalziel SR, et al. Bronchiolitis. Lancet 2022;400(10349):P392-406.
Summary
Viral bronchiolitis is the most common cause of admission to hospital for infants in high-income countries. Respiratory syncytial virus accounts for 60-80% of bronchiolitis presentations. Bronchiolitis is diagnosed clinically without the need for viral testing. Management recommendations, based predominantly on high-quality evidence, advise clinicians to support hydration and oxygenation only. Evidence suggests no benefit with use of glucocorticoids or bronchodilators, with further evidence required to support use of hypertonic saline in bronchiolitis. Evidence is scarce in the intensive care unit. Evidence suggests use of high-flow therapy in bronchiolitis is limited to rescue therapy after failure of standard subnasal oxygen only in infants who are hypoxic and does not decrease rates of intensive care unit admission or intubation. Despite systematic reviews and international clinical practice guidelines promoting supportive rather than interventional therapy, universal de-implementation of interventional care in bronchiolitis has not occurred and remains a major challenge.
(9). Monkeypox: a global wake-up call. Lancet 2022;400(10349):P337.
WHO’s declaration on July 23 that the current monkeypox outbreak constitutes a Public Health Emergency of International Concern (PHEIC) was unprecedented. It is the seventh such declaration, but the first made against the advice of a majority of the emergency committee (nine were against, six were for). Dr Tedros’ decision is a brave one. It needs to serve as a global wake-up call. The question is whether it will prompt the escalated efforts required to control the outbreak.
Dr Tedros gave three broad reasons for his decision. “We have an outbreak that has spread around the world rapidly, through new modes of transmission about which we understand too little, and which meets the criteria in the International Health Regulations.” The details make for a compelling case. So far this year, up to July 22, 16 016 cases have been reported from 75 countries. Where monkeypox is endemic, such as in DR Congo, large new outbreaks have been reported in diverse populations. Outside of west and central Africa, the outbreak is concentrated, for now, in men-who-have-sex-with-men (MSM). Why the disease’s epidemiology has changed is still unclear, as are many other aspects of the outbreak. Recent case series from non-endemic countries have shown differences in clinical features compared with previous reports. Lethargy and fever seem to be less common, and several patients have no prodromal symptoms. Skin lesions are found predominantly in genital or perianal areas. Transmission is known to occur through skin-to-skin contact, but monkeypox DNA has been found in patients’ seminal fluid-whether it represents replication-competent virus remains unknown. The rapid worldwide spread of a disease, for reasons we are unsure of, was clearly an over-riding concern for Dr Tedros. An urgent energised research effort is now needed to understand these and other issues related to the outbreak.
Countries must strengthen public health preparedness and response. Case definitions should be updated and harmonised as new data emerge, with heightened surveillance, case detection, and contact tracing. Patients need to be supported in isolation and treatment, and targeted immunisation might be needed for people at high risk of exposure. Recent experience with COVID-19 might help countries institute these measures but many health systems are at breaking point already. There is a risk too that the public is fatigued by talk of pandemics and their control. Misinformation about monkeypox has already begun to circulate. The public need to be engaged and targeted risk-communication strategies developed. Monkeypox is not COVID-19. The R0 is around 1, and transmission mechanisms are entirely different. The clade of monkeypox that seems to be responsible for the outbreak largely causes mild self-limiting illness, although patients have been admitted to hospital, mainly for pain. Ensuring wide understanding of these points is key for managing public anxiety.
Engagement among many MSM has been high since the outbreak started, and this population is-as ever-keen to take care of its health (and do their part to protect others). Countries that criminalise homosexuality and marginalise LGBTI+ communities risk both patients’ wellbeing and chances of controlling transmission. Stigma and discrimination need to be fought. It would be wrong to categorise monkeypox as a disease of MSM.
The expedited pathways for research, development, regulatory approval, and manufacturing of medical countermeasures developed for COVID-19 should be repurposed for monkeypox. A lack of diagnostic tests is hampering case identification in some countries. Tecovirimat, originally produced to treat smallpox, has been licenced by European regulators for monkeypox, but not yet by the US FDA. Other promising treatments, such as cidofovir and brincidofovir, require clinical study. A monkeypox vaccine (sold as Imvanex in Europe and Jynneos in the USA) has been approved, but supplies of both treatments and vaccines are extremely limited. WHO will have to take a much more muscular approach to ensure global access and avoid the inequities of the COVID-19 response.
Whether or not you agree with WHO’s decision, there has undoubtedly been a missed opportunity. Monkeypox is not new. It has been causing illness and death in large numbers for decades. Specialists have long called for affordable countermeasures, strengthened surveillance, and more study. But like Ebola and Zika, monkeypox only commands global attention when it hits high-income countries with predominantly White populations. As a result, the window of opportunity to prevent monkeypox becoming established in communities worldwide is closing. Now is a key moment. It warrants the strongest medical, scientific, and political global effort.
(10). Puja Mehta et al. Baricitinib in COVID-19: a coming-of-age from artificial intelligence to reducing mortality. Lancet 2022;400(10349):P338-339.
Immunomodulatory therapies targeting excessive host immune responses,1 vaccination, and immunity from natural infection have changed the course of the COVID-19 pandemic. However, the rapid emergence of SARS-CoV-2 variants has stymied progress towards ending the pandemic. An unmet need remains for accessible therapies that reduce mortality. In The Lancet, the RECOVERY Collaborative Group assessed the use of baricitinib, a Janus kinase (JAK) inhibitor, for the treatment of patients hospitalised with COVID-19, in the randomised, controlled, open-label platform trial (Randomised Evaluation of COVID-19 Therapy [RECOVERY]).2 The potential use of baricitinib in COVID-19 was first identified from an artificial-intelligence-enabled drug discovery algorithm.3 Baricitinib can suppress multiple cytokine-signalling pathways simultaneously and impede viral propagation through inhibition of numb-associated kinases important for clarthrin-mediated endocytosis.4
RECOVERY was conducted in the UK and 8156 patients (mean age 58.1 years, 66% male, 34% female, 80% White) were randomly allocated to receive usual care plus baricitinib or usual care alone (which included corticosteroids). Patients receiving baricitinib plus usual care had a significant reduction in the primary outcome of 28-day mortality. Of the patients randomly assigned, 514 (12%) of 4148 patients in the baricitinib group died compared with 546 (14%) of 4008 patients in the usual care group (age-adjusted rate ratio 0.87; 95% CI 0.77-0.99; p=0.028) with a number-needed-to-treat (NNT) to prevent one additional death of 50. The authors did a meta-analysis of all nine completed randomised trials of JAK inhibitors for COVID-19 including the results of this study and found a proportional 20% reduction in mortality associated with JAK inhibitors. RECOVERY is the largest trial of baricitinib for COVID-19 and confirms the findings of previous studies thus validating the addition of this drug to the arsenal of COVID-19 therapies.
View related content for this article
The RECOVERY trial is an international trial but baricitinib was only evaluated in the UK and these findings might be less generalisable to populations with different demographics and higher prevalence of HIV and latent tuberculosis (<1% enrolled). Baricitinib is an oral agent and of lower cost in many countries compared with intravenous tocilizumab, which also confers a mortality benefit.5 Baricitinib could be more accessible in lower-resourced settings, although its effects on non-COVID-19 infections in these countries needs better characterisation. The open-label design of RECOVERY has been criticised for risk of bias;6 it is, however, unlikely to influence an unambiguous primary outcome of mortality. The adaptive design has yielded timely and invaluable results amid the challenges of doing practice-changing research during a pandemic.
Meta-analyses of previous JAK inhibition trials in patients with COVID-19, as well as analyses restricted to baricitinib-only trials (n=4, including RECOVERY), strongly support targeting the JAK-STAT (signal transducer and activator of transcription) axis in COVID-19, although mechanistically the influence of JAK isoform selectivity is unclear. The mortality risk reduction with baricitinib in RECOVERY was smaller than anticipated, which is probably explained by a broader eligibility in RECOVERY compared with other studies of baricitinib for hospitalised patients with COVID-19 (appendix), which all mandated hypoxaemia. COV-BARRIER showed a 5% absolute reduction in mortality at both day 28 and 60, NNT 20,7 and included an entry requirement of evidence of inflammation, although had lower thresholds than the tocilizumab group of RECOVERY (hypoxaemia and C-reactive protein ≥75 mg/L).5 Subgroup analyses in both ACTT-28 and COV-BARRIER,7 suggested greater benefit of baricitinib in more severe disease.
WHO’s living guidance recommends baricitinib for patients with severe or critical COVID-19 in combination with corticosteroids.9 The precise positioning of baricitinib is unclear, but expanding choice stimulates questions regarding personalised immunomodulation regimens, incorporating side-effect profiles, routes of administration, cost, and patient comorbidities. The ACTT-4 trial did not show a difference in outcomes between the baricitinib and dexamethasone groups, although dexamethasone was associated with significantly more adverse events.10 Available evidence is insufficient to suggest that baricitinib would routinely replace dexamethasone; however, it could be a viable steroid-sparing option in patients at high risk of glucocorticoid side-effects. In RECOVERY, 23% of patients in both groups received tocilizumab, yet the benefits of baricitinib were consistent irrespective of co-administration. Data are insufficient for further interpretation, but notably both agents might increase the risk of gastrointestinal perforation (also applicable to concomitant corticosteroids), and cause pharmacodynamic C-reactive protein suppression. JAK inhibitors have a broader but shorter immunosuppressive effect than selective cytokine blockade (ie, in adults, 12-h half-life for baricitinib compared with 11-13 days for tocilizumab). Where there is a higher risk of secondary infections, baricitinib’s broader immunosuppressive effect might be considered an advantage given the faster onset of action or a disadvantage with greater dampening of host defences. However, faster wash-out when discontinued is of undoubted value. Baricitinib can be dose-adjusted in renal impairment and delivered by nasogastric tube in patients who are ventilated. Further research is awaited to establish whether baricitinib might also have a role in other hyperinflammatory disorders of immune dysregulation, including sepsis and other viral epidemics (eg, dengue syndrome).11
Safety data for baricitinib is reassuring, especially related to thrombotic events, probably owing to the limited treatment duration in patients with COVID-19 who are anticoagulated. This finding differs from chronic JAK inhibitor dosing in rheumatoid arthritis studies, where safety signals were detected.12 Guidelines do not recommend JAK inhibitors in pregancy13 and pharmacokinetic studies suggest that the half-life of baricitinib in children is substantially shorter than in adults, requiring dosing up to four times per day.14 Tocilizumab might be preferred in patients who are pregnant or paediatric, given greater clinical experience and convenient dosing respectively.
From early days in artificial intelligence algorithms to pharmacogenomic predictions15 we now have compelling efficacy and safety data for baricitinib in patients with COVID-19. Baricitinib’s evolution is an exemplar of modern-day candidate selection, proof-of-concept testing, and drug repurposing, serving as a template for drug discovery-a powerful tool in future pandemic preparedness.
(11). Hans-Christoph D. The Role of Aspirin Today.
https://www.medscape.com/viewarticle/976621
We are celebrating the 125th anniversary of aspirin. Aspirin was first synthesized in Wuppertal, Germany, a city which is only 40 km from my location, by Felix Hoffmann. Hoffmann was searching for a new drug for his father who suffered from severe joint pain, and the available drugs at that time had terrible adverse events. This prompted him to work on a new drug, which was later called aspirin acetylsalicylic acid.
Aspirin has been used very successfully to the present day as therapy for joint pain or arthritis. But as you know, it’s also effective in headaches, in particular, tension-type headache. I think it’s one of the most used drugs in the world for the treatment of acute migraine attacks.
It’s also available in some European countries in intravenous form for the treatment of severe migraine attacks or in the emergency room, and it’s as effective as subcutaneous sumatriptan. It’s also an effective migraine preventive drug in a dose of 300 mg/d.
Discovering Aspirin’s Antiplatelet Activity
There was an interesting observation by a dentist in the 1930s, who noted bleeding when he extracted teeth in people who took aspirin for joint pain. When he started to ask his patients about possible bleeding complications and vascular events, he observed that people who took aspirin didn’t have coronary myocardial infarctions.
It took a long time for people to discover that aspirin is not only a pain medication but also an antiplatelet agent. The first randomized study which showed that aspirin is effective in secondary prevention after myocardial infarction was published in 1974 in The New England Journal of Medicine. In 1980, aspirin was approved by the US Food and Drug Administration for the secondary prevention of stroke and in 1984 for secondary prevention after myocardial infarction.
A History of Efficacy
Aspirin also has a proven role in the secondary prevention of transient ischemic attack and ischemic stroke. Given early, it reduces the risk for a recurrent vascular event by 50% and long-term, compared with placebo, by 20%.
Interestingly, the doses are different in different areas of the world. In the United States, it’s either 81 mg or 325 mg. In Europe, it’s usually 100 mg. Until a few years ago, there was no single trial which used 100 mg of aspirin compared with placebo for the secondary prevention of stroke.
If we look at dual antiplatelet therapy, the combination of aspirin and clopidogrel was not superior to aspirin alone or clopidogrel alone for long-term prevention, but the combination of dipyridamole and aspirin and the combination of cilostazol and aspirin were superior to aspirin alone for secondary stroke prevention. Short-term, within the first 30 days, the combination of aspirin and clopidogrel and the combination of ticagrelor and aspirin is superior to monotherapy but also have an increased risk for bleeding.
People with atrial fibrillation or embolic strokes need to be anticoagulated, but the addition of aspirin to anticoagulation does not increase efficacy, it only increases the risk for bleeding.
In people above the age of 75 years who have to take aspirin, there is an increased risk for upper gastrointestinal bleeding. These patients should, in addition, receive proton pump inhibitors.
The use of aspirin for the primary prevention of vascular events was promoted for almost 50 years all over the world, but in the last 5 years, a number of randomized trials clearly showed that aspirin is not effective compared with placebo in the primary prevention of vascular event stroke, myocardial infarction, and vascular death. It only increases the risk for bleeding.
So it’s a clear separation. Aspirin should not be used for primary prevention of vascular events, but it should be used in basically everyone who doesn’t have contraindications for secondary prevention of vascular events and vascular death.
A drug that is 125 years old is also still one of the most used and affordable drugs all around the world. It’s highly effective and has only a small risk for major bleeding complications. It’s really time to celebrate aspirin for this achievement.
(12). Chuan De Foo. Isolation facilities for covid-19: towards a person centred approach. BMJ 2022;378:e069558.
Isolation facilities for covid-19
An isolation facility is a dedicated place where people who test positive for covid-19 receive essential care and are provided with daily necessities, such as food, safe drinking water, and toiletries, as they recover.5 “Mandatory” isolation means that everyone who meets certain criteria-typically a positive test within a defined period, even if asymptomatic or with mild clinical symptoms that do not require hospital admission-must be confined until they meet the criteria for discharge, are referred to other facilities, or die. This is different from quarantine (which isolates, temporarily, those considered at risk of spreading infection but not known to be infected), but both functions might sometimes be combined, as in New Zealand with its facilities for “managed isolation and quarantine.”
Key messages
Isolation facilities have historically been used to limit community spread of infectious diseases
Governments revisited the idea to curb covid-19 transmission and safeguard health systems
Isolation facilities were a feasible alternative when self-isolation was not possible, but reports of inequitable and unfair requirements regarding mandatory isolation have surfaced
They must be staffed by a trained, protected, and well equipped interdisciplinary workforce and oriented to person centred care
Isolation facilities are but one component of a comprehensive public health strategy
(13). Wu RL, et al. Low-Dose Subcutaneous or Intravenous Monoclonal Antibody to Prevent Malaria. N Engl J Med. 2022; 387:397-407.
New approaches for the prevention and elimination of malaria, a leading cause of illness and death among infants and young children globally, are needed.
Methods
We conducted a phase 1 clinical trial to assess the safety and pharmacokinetics of L9LS, a next-generation antimalarial monoclonal antibody, and its protective efficacy against controlled human malaria infection in healthy adults who had never had malaria or received a vaccine for malaria. The participants received L9LS either intravenously or subcutaneously at a dose of 1 mg, 5 mg, or 20 mg per kilogram of body weight. Within 2 to 6 weeks after the administration of L9LS, both the participants who received L9LS and the control participants underwent controlled human malaria infection in which they were exposed to mosquitoes carrying Plasmodium falciparum (3D7 strain).
Results
No safety concerns were identified. L9LS had an estimated half-life of 56 days, and it had dose linearity, with the highest mean (±SD) maximum serum concentration (Cmax) of 914.2±146.5 μg per milliliter observed in participants who had received 20 mg per kilogram intravenously and the lowest mean Cmax of 41.5±4.7 μg per milliliter observed in those who had received 1 mg per kilogram intravenously; the mean Cmax was 164.8±31.1 in the participants who had received 5 mg per kilogram intravenously and 68.9±22.3 in those who had received 5 mg per kilogram subcutaneously. A total of 17 L9LS recipients and 6 control participants underwent controlled human malaria infection. Of the 17 participants who received a single dose of L9LS, 15 (88%) were protected after controlled human malaria infection. Parasitemia did not develop in any of the participants who received 5 or 20 mg per kilogram of intravenous L9LS. Parasitemia developed in 1 of 5 participants who received 1 mg per kilogram intravenously, 1 of 5 participants who received 5 mg per kilogram subcutaneously, and all 6 control participants through 21 days after the controlled human malaria infection. Protection conferred by L9LS was seen at serum concentrations as low as 9.2 μg per milliliter.
Conclusions
In this small trial, L9LS administered intravenously or subcutaneously protected recipients against malaria after controlled infection, without evident safety concerns
