Rationalise and Restrict Antibiotic Use by Utilizing A Proactive Justification Form and Comparing with Earlier Antibiotic Usage in The Same Paediatric Unit in A Tertiary Care Centre
K. Banupriyanga, D. Senguttuvan, Suresh Chelliah*
Department of Paediatrics, Kauvery Hospital, Trichy, Tamilnadu
*Correspondence: Email: chelliah.suresh@yahoo.co.in
Abstract
Background: The objective of the study was to find out whether introduction of antibiotic justification form restricts and rationalises antibiotic usage.
Methods: This Quasi-experimental study was conducted in paediatric ward at our hospital. We evaluated the efficacy of an intervention, wherein paediatricians were made to fill a justification form before starting on antibiotics. Antimicrobial usage percentage over a year after introduction of justification form was compared with that in the year preceding it. Pre- and post-intervention periods were June 2017 to March 2018 and June 2018 to March 2019, respectively.
Results: After introduction of justification form, usage of antibiotics decreased by 26.9% (p < 0.001), with increase in de-escalation (p < 0.001) and decrease in duration (p < 0.001).
Conclusion: Implementation of antibiotic justification form, a simple and effective antibiotic stewardship intervention in our paediatric unit, led to substantial reduction in antibiotic usage and duration, with increase in rationalisation and de-escalation rates.
Keywords: Antibiotics, Antimicrobial resistance, Antibiotic stewardship
Background
Antibiotics are among the drugs most commonly prescribed to children in hospital and community settings [1–3]. It has been reported that the average proportion of children in hospital settings who receive at least one antibiotic is between 33% and 78% [4–8].
Unfortunately, a substantial proportion of antimicrobial prescribing is unnecessary or inappropriate, including selection, dose and duration [9,4]. Overuse and misuse of antibiotics are primary drives of antibiotic resistance, which is a rapidly growing global health threat [10].
India is among the nations with highest burden of bacterial infections. An estimated 410,000 children aged five years or less die from pneumonia in India annually; accounting for almost 25% of all child deaths in India. The crude mortality from infectious diseases in India today is 417 per 100,000 persons [11].
The impact of Antimicrobial Resistance (AMR) is higher in the Indian setting. Resistance against all groups of antimicrobials, both first line and last resort drugs, is on the rise. AMR has emerged as a public health concern, especially in the light of the fact that development of newer classes of antibiotics has been slow [11].
An indicator of rising tide of AMR in India is the rapidly increasing proportion of isolates of Staphylococcus aureus that are resistant to methicillin. In 2008, about 29% of isolates were Methicillin Resistant Staphylococcus aureus (MRSA), by 2014, this had risen to 47% [12].
Investigating and monitoring the consumption of antimicrobials in hospitals is necessary to encourage prudent use of these drugs. Antibiotic stewardship programs are one of the core strategies that can be used to address antibiotic overuse and resistance.
Antibiotic Stewardship (ASP) is a multidisciplinary programme which delivers a package of interventions to improve appropriate use of antibiotics, monitoring and evaluating the impact of the programme on improvement in antibiotic prescription practices and reduction of resistance levels [13]. Antibiotic stewardship can be done by different means; restraining the prescription, switching to a narrower spectrum or stopping antibiotics when not needed [14,15].
For several years, antibiotic stewardship efforts were focused on adult populations. Data on the effects of ASPs in the paediatric population are still scarce. A systematic review by Smithet al., using PubMed identified only nine studies that evaluated outcomes associated with formalised paediatric ASPs originating from four centres [16], where as in adults, a systematic review conducted in 2013 to study the evidence for effects of inpatient ASPs evaluated 37 articles [17].
In this study, we evaluated the efficacy of an intervention, wherein paediatricians were made to fill a justification form before starting on antibiotics. Antimicrobial usage pattern over the next year was compared with that in the one year preceding the introduction of justification form.
Methods
This is a Quasi-experimental study, conducted at paediatrics department, Kauvery Speciality Hospitals, Trichy. Children who were admitted to the paediatric ward from June 2018 to March 2019 were selected by systematic random sampling and enrolled in study as post intervention group after obtaining informed consent from the parents. Children who were admitted to the paediatric ward previous year, during the same time period June 2017 to March 2018 were taken as pre intervention group, after obtaining permission from institutional ethics committee. Children admitted in PICU were excluded.
Paediatricians were asked to fill the justification form within 24 hours of starting an antibiotic after taking cultures. Culture reports were notified to the treating paediatrician within 48 to 72 h. If the consultant decides to continue antibiotic, justification has to be written in the form, which was reviewed again on day seven or at discharge. Any decision to continue antibiotic further has to be justified in the form provided.
Data was processed using SPSS v. 20.0. Independent T-test and Chi Square test were used to arrive at statistical significance.
Results
Table 1 shows similar pattern of illness in both the groups over the two time periods studied.
Table 1. Profile of patients.
| Diagnosis | Group Pre-intervention n (%) | Group Post-intervention n (%) |
| Enteric fever | 17 (4.5) | 10 (2.6) |
| Suspected enteric | 24 (6.3) | 32 (8.4) |
| Urinary tract infection | 13 (3.4) | 7 (1.8) |
| Suspected UTI | 15 (3.9) | 6 (1.6) |
| Pneumonia | 27 (7.1) | 24 (6.3) |
| Injuries | 17 (4.5) | 13 (3.4) |
| Dengue fever | 48 (12.6) | 62 (16.3) |
| HINI | 6 (1.6) | 23 (6.1) |
| Appendicitis | 11 (2.9) | 9 (2.4) |
| Pharyngitis/Otitis media | 16 (4.2) | 4 (1.1) |
| LRI/WALRI/Bronchitis | 48 (12.6) | 40 (10.5) |
| Nephrotic syndrome | 4 (1.1) | 6 (1.6) |
| Scrub typhus | 10 (2.6) | 9 (2.4) |
| PUO | 7 (1.8) | 4 (1.1) |
| Febrile seizure | 10 (2.6) | 10 (2.6) |
| Seizure disorder | 3 (0.8) | 2 (0.5) |
| Viral fever | 10 (2.6) | 12 (3.2) |
| AGE (Acute Gastro Enteritis) | 47 (12.4) | 53 (13.9) |
| AFI (Acute Febrile Illness) | 44 (11.6) | 36 (9.5) |
| Rheumatic heart disease | 1 (0.3) | 1 (0.3) |
| Others* | 2 (0.5) | 17 (4.5) |
*Others: Thalassemia, Leukaemia, Ectodermal dysplasia, Short stature.
Fig.1 shows infective etiology as a reason for starting antibiotics was 55.3% in the pre-intervention group and 44.7% in the post-intervention group. An antibiotic started for non-infective etiologies was 3.7% in both the groups, 45.3% of the study population during the year 2018–2019 (post-intervention group) were not started on antibiotics as compared to 17.6% in the year 2017–2018 (pre-intervention). In the pre-intervention group, 23.4% were given antibiotics without a valid, documented reason which decreased to 6.3% post introduction of justification form.
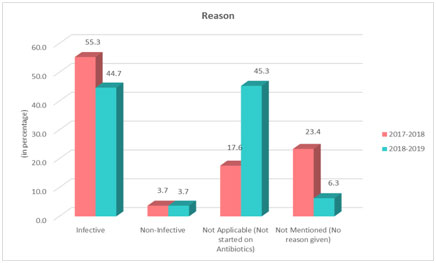
Fig 1. Reason for initiating antibiotics.
Table 2 illustrates that Antibiotics were started for 82.4% of study population in pre-intervention group which decreased to 55.5% post introduction of justification form. The difference is statistically significant with p < 0.001.
Table 2. Antibiotic usage.
| Antibiotics | Pre-intervention | Post-intervention | |
| Started | 313(82.4%) | 211(55.5%) | |
| Not started | 67(17.6%) | 169(44.5%) | p < 0.001 |
| Total | 380 (100%) | 380 (100%) |
Fig.2 shows 17.1% of patients had de-escalation of antibiotics post introduction of justification form as compared to 1.9% in the pre-intervention period. The difference was statistically significant p < 0.001.
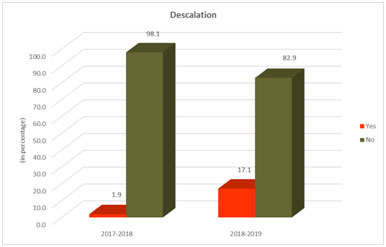
Fig. 2. De-escalation of antibiotics.
Table 3 shows decrease in duration of antibiotics post introduction of antibiotic justification form as compared to the pre-intervention period. There was a significant decrease in duration of therapy in the study year as compared to the previous year. The difference was statistically significant with p < 0.001.
Table 3. Duration of antibiotic usage.
| Duration of antibiotics (days) | Group | |
| Pre-intervention | Post-intervention | |
| <5 | 18 (5.8) | 36 (17.1) |
| 5–7 | 262 (83.7) | 143 (67.8) |
| >7 | 33 (10.5) | 32 (15.2) |
| >Total | 313 (100.0) | 211 (100.0) |
| >P value | <0.001 | |
Discussion
Out of 380 in the pre-intervention group, 313 children were started on antibiotics which is 82.4% whereas in the post-intervention group, 211 out of 380 children were started on antibiotics, which is 55.5%. The post intervention group had statistically significant (p < 0.001) decrease in antibiotic usage. Similar results were obtained by Bhullar et al., in a PICU at Hyderabad during 2013 to 2014 and Di Pentima et al., at DuPont Hospital for children during 2001–2007 [19,20].
The demographic profile and disease spectrum among both the groups being comparable, the reduction in initial usage and de-escalation of antibiotics may be attributed to the awareness created by the justification form. The written commitment which the paediatrician had to make, was probably the most important factor in reducing antibiotic usage.
Reason for starting antibiotics was not mentioned for 89 (23.4%) children in the pre-intervention group which reduced to 24 (6.3%) children in the post-intervention group.
De-escalation of antibiotics was done for 36 (17.1%) children in the post-intervention group as compared to 6 (1.9%) children in pre-intervention group, which is statistically significant (p < 0.001). Duration of antibiotic use has significantly reduced (p < 0.001) in the post intervention group.
Our study has the following limitations that restricted antibiotics were not separately studied. Children admitted in PICU were not included in the study.
Conclusion
We conclude that implementation of antibiotic justification form which is a simple and effective antibiotic stewardship intervention, led to a substantial reduction in antibiotic usage and duration with increase in rationalisation and de-escalation rates.
It is a simple, no cost intervention with vast implications in any clinical setup, thereby reducing antibiotic resistance.
This form makes antibiotic usage audit by peers simple. This can be extrapolated to the entire hospital setting including outpatient clinic.
Stage V: Analysis and reporting
Data collected are analyzed. Results are compared with criteria and standards.
References
- Zaoutis TE. Variability in antibiotic use at children’s hospitals. Pediatrics. 2010;126:1067-1073.
- Ashiru-Oredope D, Hopkins S. English surveillance programme for antimicrobial utilization and resistance oversight group. antimicrobial stewardship: English surveillance programme for antimicrobial utilization and resistance (ESPAUR). J Antimicrob Chemother. 2013;68:2421-2423.
- Bielicki J, Drapier N, Zaoutis T, Tsolia M, Sharland M. ARPEC Project Group Members. Variation in paediatric hospital antibiotic guidelines in Europe. Arch Dis Child. 2016;101:72-76.
- Levy ER, Swami S, Dubois SG, Wendt R, Banerjee R. Rates and appropriateness of antimicrobial prescribing at an academic children’s hospital, 2007–2010. Infect Control Hosp Epidemiol. 2012;33:346-353.
- Pakyz AL, Gurgle HE, Ibrahim OM, Oinonen MJ, Polk RE. Trends in antibacterial use in hospitalized pediatric patients in United States academichealth centers. Infect Control Hosp Epidemiol. 2009;30:600-603.
- Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, Levine GL, Goldmann DA, Jarvis WR. Use of antimicrobial agents in United States neonatal and paediatric intensive care patients. Pediatr Infect Dis J. 2005;24:766-773.
- Rutledge-Taylor K, Matlow A, Gravel D, et al. Canadian nosocomial infection surveillance program. Canadian nosocomial infection surveillance program: a point prevalence survey of health care associated infections in Canadian pediatric inpatients. Am J Infect Control. 2012;40:491-496.
- Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H. ARPEC project group. The worldwide antibiotic resistance and prescribing in European children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016.
- Dellit TH, Owens RC, McGowan JE, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
- Magsarili HK, Girotto JE, Bennett NJ, et al. Making a case for paediatric antimicrobial stewardship programs. Pharmacotherapy. 2015;35(11) :1026-1036.
- Inter-Ministerial Review Meeting on Antimicrobial Resistance – Antimicrobial resistance and containment in India, November 2016:4-14.
- Center for Disease Dynamics Economics and Policy. Resistance Map. (http://resistancemap.cddep.org/resmap/c/in/India, accessed 20 June, 2016).
- Chandy SJ, Michael JS, Veeraraghavan B, Abraham OC, Bachhav SS, Kshirsagar NA. ICMR programme on antibiotic stewardship, prevention of infection & control (ASPIC). Indian J Med Res. 2014.
- Annual Epidemiological Report on Communicable Diseases in Europe 2008: Report on the state of communicable Diseases in the EU and EEA/EFTA countries. European Centre for Disease Prevention and control; 2008.
- Vogelaers D, De Bels D, Foret F, Cran S, Gilbert E, Schoonheydt K. Patterns of antimicrobial therapy in severe nosocomial infections: empiric choices, proportion of appropriate therapy, and adaptation rates – a multicentre observational survey in critically ill patients. Int J Antimicrob Agents.2010;35:375-381.
- Smith MJ, Gerber JS, Hersh AL. Inpatient antimicrobial stewardship in paediatrics: A systematic review. J Pediatr Infect Dis Soc.2015;4(4):e127-e135.
- Wagner B, Filice GA, Drekonja D, et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol. 2014;35:1209-1228.
- Magsarili HK, Girotto JE, Bennett NJ, et al. Making a case for pediatric antimicrobial stewardship programs. Pharmacotherapy. 2015;35(11):1026-1036.
- Bhullar HS, Shaikh FA, Deepak R, Poddutoor PK, Chirla D.Antimicrobial justification form for restricting antibiotic use in a paediatric intensive care unit. Indian Paediatr. 2016;53:290-291.
- [20]. Newland JG, Stach LM, Hedican EB, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1:179-186.
