Bini Susan Isaac*
Clinical Pharmacist, Kauvery Hospital, Salem, India
*Correspondence: +91 9497052726; clinicalpharma.khs@kauvery.in
A novel direct thrombin inhibitor from tick salivary transcriptoms: A review
Abstract
The tick midgut and salivary glands represent the primary organs for pathogen acquisition and transmission, respectively. Specifically, the midgut is the first organ to have contact with pathogens during the blood meal uptake, while salivary glands along with their secretions play a crucial role in pathogen transmission to the host. Currently, there is little data about pathogen composition and prevalence in Ixodes ricinus midgut and salivary glands. The present study investigated the presence of 32 pathogen species in the midgut and salivary glands of unfed I. ricinus males and females using high-throughput microfuidic real-time PCR. Such an approach is important for enriching the knowledge about pathogen distribution in distinct tick organs which should lead to a better understanding I. ricinus borne disease epidemiology.
Keywords: Thrombin inhibitor (DTI), dual antiplatelet therapy (DAPT), unfractionated heparin (UFH)
Background
During percutaneous coronary intervention (PCI), balloon angioplasty is frequently followed by stent implantation. The procedure causes extensive endothelial disruption and injury, leading to an intense burst of thrombin generation [1]. As such, patients are routinely pre-treated with dual antiplatelet therapy (DAPT) and administered an injectable anticoagulant, commonly unfractionated heparin (UFH), during the PCI procedure [2]. The limitations of UFH include major bleeding, heparin-induced thrombocytopenia (HIT) and the need for coagulation monitoring due to its unpredictable pharmacokinetics. However, UFH remains one of the most widely used parenteral anticoagulants due to low cost the wealth of clinical experience accumulated in more than eight decades of use [3]. To overcome some of UFH’s disadvantages, an injectable direct thrombin inhibitor (DTI), bivalirudin, was developed as an alternative to UFH in PCI [4]. Although bivalirudin is more expensive (~$400 to $600 per PCI without post-procedural infusion) [5], initial randomised trials showed that bivalirudin was associated with similar antithrombotic effificacy but less bleeding when compared with a combination of UFH and a platelet glycoprotein IIb/IIIa inhibitor [6]. However, protocol-mandated use ofglycoprotein IIb/IIIa inhibitors in the UFH arm of these trials could have contributed to the higher bleeding rates among patients randomised to UFH.
Discussion
Haematophagous animals such as leeches, mosquitoes, ticks, tsetse flies and others are rich sources of antithrombotic agents. Molecules such as hirudin from the medicinal leech, anophelin from mosquitoes madanin from ticks and tsetse thrombin inhibitor from these flies are potent and selective thrombin inhibitors that are highly amenable to customisation as synthetic inhibitors with improved properties [7].
Ticks
Ticks are vectors of a large number of pathogenic microorganisms including bacteria, protozoa and viruses, which cause serious diseases in both humans and animals. Ixodes ricinus is the predominant tick species in Europe and is recognised as the primary vector of infectious diseases in humans, including Lyme borreliosis caused by Borrelia burgdorferi (sensu lato). Over the past decade, the number of studies characterizing pathogens carried by I. ricinus has considerably increased, enriching the wealth of information on I. ricinus pathogen composition. It is also important to note that I. ricinus is regularly found to be co-infected by several pathogenic agents. This information is crucial considering that different co-infection combinations in humans and animals are highly likely to alter disease symptoms and severity.
Pathogen acquisition, development and transmission
Salivary glands may be co-infected by several different pathogens which can then be transmitted to the vertebrate host during blood-feeding along with salivary secretions. Moreover, the tick midgut presents a pivotal microbial entry point and determines pathogen colonization and survival in the tick. The common pathogenic life-cycle within the tick vector starts with ingestion of the infected host blood, pathogen migration through the gut to the haemocoel, transport into and infection of the salivary glands, then transmission to another host during the next blood meal. This pathway can vary depending on the pathogenic agent. We have previously characterised a new class of DTIs, variegin and other variegin-like peptides, from the salivary gland of the tropical bont tick (Amblyomma variegatum). Variegin, a 32-residue peptide, binds to both thrombin’s active site and exosite-I. Variegin inhibits thrombin more potently than bivalirudin by sixfold and demonstrates superior selectivity for thrombin. Bivalirudin has a half-life of 25 min and must therefore be administered as a continuous infusion during PCI as the majority of these procedures last ~30 min to an hour. Variegin has a half-life of 52.3 ± 4.4 min and may potentially be given as a single bolus for peri-PCI anticoagulation.
Porcine Ex Vivo Stent Thrombosis
Better efficacy & safety in combination with DAPT
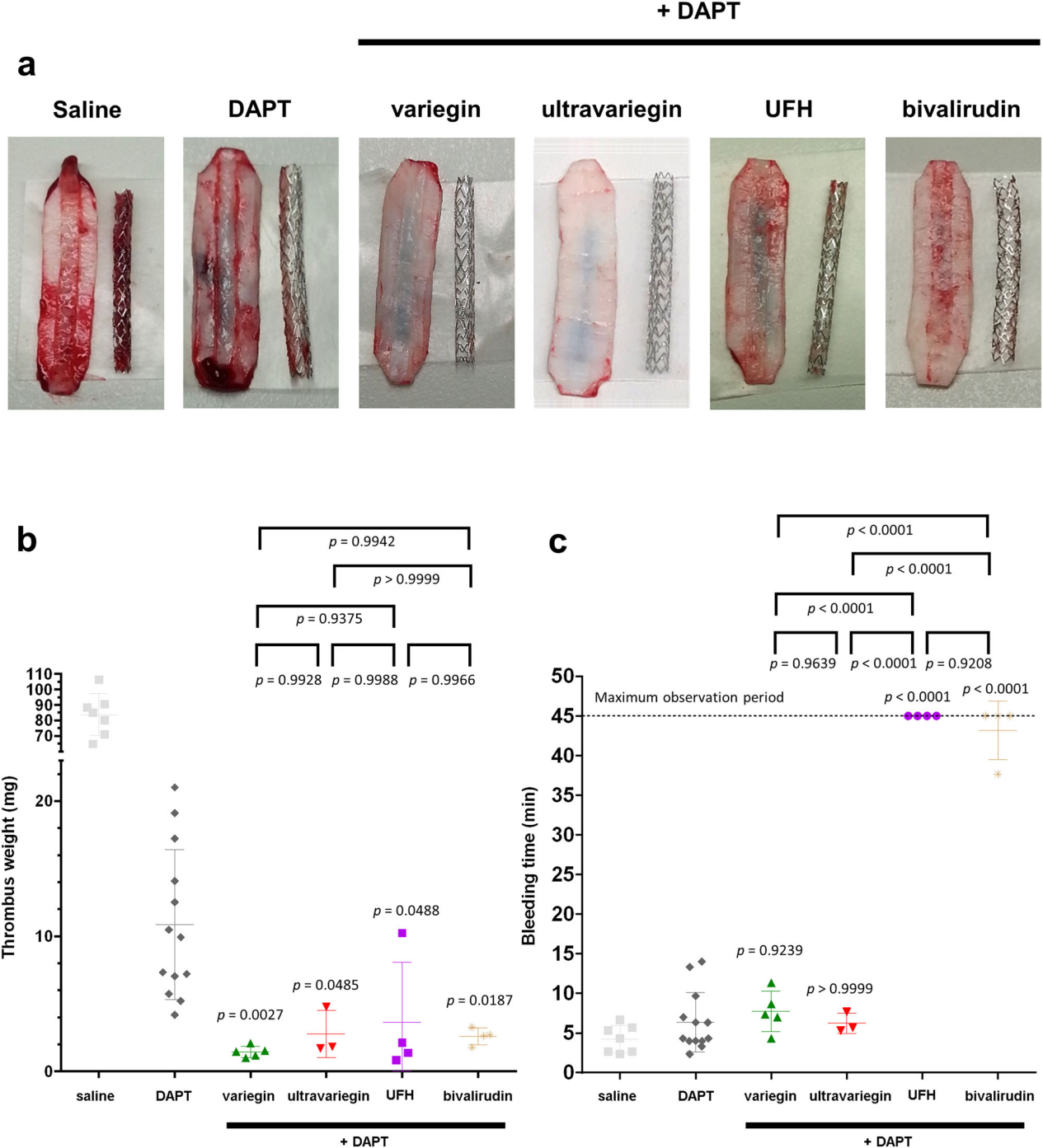
Efficacy and safety were determined using ex vivo stent thrombosis and superficial ear vein bleeding models, respectively. All pigs were administered 300 mg aspirin and 180 mg ticagrelor orally (DAPT regimen) 16 h prior to the experiments. a Representative photographs showing thrombi formed oncoronary stents and endothelium-denuded pig aorta strips in pigs administered with DAPT only (n = 13), DAPT with 0.1 mg/kg variegin (i.v. bolus, n = 5), DAPT with 0.025 mg/kg ultravariegin (i.v. bolus, n = 3), DAPT with 100 u/kg UFH (i.v. bolus, n = 4), and DAPT with 0.75 mg/kg (i.v. bolus) plus 1.75 mg/kg/h (i.v. continuous infusion) bivalirudin (n = 4). Photograph from a pig receiving saline without DAPT, as depicted in Fig. 1a, is reproduced here for comparison. Experiments were repeated the indicated n number of animals independently with similar results. b Total weight of thrombi (one-way ANOVA P = 0.0007). c Bleeding time (one-way ANOVA P < 0.0001). Multiplicity adjusted P values for post hoc Tukey’s multiple comparisons between respective anticoagulants with DAPT are indicated immediately above each treatment. Multiple comparison among anticoagulants were indicated on top of the plot. Data shown are mean ± SD.
The low dose of variegin and ultravariegin reduced thrombus formation from DAPT alone by 87% and 75%, respectively (Fig. 1a, b). In comparison, the clinically approved dose of UFH and bivalirudin reduced thrombus formation from DAPT alone by 66% and 76%, respectively (Fig. 1a, b). Low-dose variegin (7.7 ± 2.6 min) or ultravariegin (6.2 ± 1.3 min) with DAPT did not increase the bleeding time compared to DAPT without anticoagulation (6.4 ± 3.7 min). In contrast, UFH or bivalirudin with DAPT both resulted in the maximally observed bleeding time of 45 min. Figure 1c shows that, in the presence of DAPT, UFH or bivalirudin resulted in at least fivefold higher bleeding times than either variegin or ultravariegin. Taken together, on a background of DAPT, low-dose variegin or ultravariegin have marginally greater efficacy in preventing stent thrombosis than UFH or bivalirudin but with substantially less bleeding [8].
Ticks, other medical applications
The last three decades of research into tick salivary components have revealed several proteins with important pharmacological and immunological activities. medical applications such as immunomodulation, inhibition of hemostasis, against tumor growth and inflammation. Ticks, in turn, secrete saliva at the bite site that contains proteinaceous molecules (enzymes, lipocalins, protease inhibitors, etc.), non-proteinaceous molecules (prostaglandins, prostacyclins), and ncRNAs (miRNAs and lncRNAs).
References
[1]. Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000;83:361-6.
[2]. Franchi F, Rollini F, Angiolillo DJ. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol. 2017;14:361-79.
[3]. Widimsky P. Is bivalirudin just an expensive heparin? Eur. Heart J. 2016;37:1321-4.
[4]. Mackman N, Bergmeier W, Stouffer GA, et al. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discovery. 2020;19:333-52.
[5]. Hillegass WB, Bradford GS. Risk guided use of the direct thrombin inhibitor bivalirudin: insights from recent trials and analyses. J Thorac Dis. 2016;8:E1034-40.
[6]. Stone GW, et al. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. J Am Med Assoc. 2007;298:2497-506.
[7]. Koh CY, Kini RM. Molecular diversity of anticoagulants from haematophagous animals. Thromb Haemost. 2009;102:437-453.
[8]. Cho Yeow Koh, Shih N, et al. Efficacy and safety of next-generation tick transcriptome-derived direct thrombin inhibitors. 2021;12:6912.

Dr. Bini Susan Isaac
Clinical Pharmacist
