Cefepime – Tazobactum – induced fluid – filled blisters (Bullous lesions): A case report
K. Sathyapriya
Senior Clinical Pharmacist, Kauvery Hospital, Electronic City, Bengaluru
Background
In the current era, the bacteria developing resistance to antibiotics has increased. The Multi Drug Resistant GNB strains like Amp C, ESBL, OXA and Carbapenamase are emerging as major health problems in the world. In recent days, E. coli and Klebseilla pneumonia have also shown resistance to Carbapenems. Cefepime 4th generation cephalosporin, with broad spectrum activity, is sensitive against Amp C and OXA, but unstable towards the ESBL producing organisms. Tazobactum, a triazolylmethyl penicillanic acid sulphone, is a potent inhibitor of plasmid mediated enzymes which commonly show resistance to penicillin’s. Cephalosporin’s have minimal activity when used alone. In order to eradicate these kinds of resistance, the novel combination of Cefepime and Tazobactum was discovered and is expected to cover all three major resistance mechanisms. The combination of Cefepime + Tazobactum will show synergistic effect by expanding the spectrum of activity against many beta-Iactamase producing bacterial strains. Cefepime- Tazobactum is a time dependent (T > MIC) broad spectrum antibiotic where prolonged infusion will show more bactericidal activity than intermittent infusion.
This combination is used for the following indication like:
- Complicated and uncomplicated UTI
- Uncomplicated skin and skin structure infections
- Complicated Intra-abdominal Infections
Case Study
A 66-year aged female patient was admitted to ER with complaints of cough and expectoration. Patient was a known case of meningo-encephalopathy (Tubercular- treated, Carcinomatosis, with Hydrocephalus), status post VP shunt, and grade 4 bed sore with Tracheotomy and on PEG feeds. She was admitted to our hospital for further management. Patient was started with Inj. Micropime 1.125 gm IV BD, Inj. Optineuron OD, Tab. Pan 40mg OD, Tab. Levipil 500mg, Tab. Folvite 5mg, Tab. Pyridoxine 40mg. The patient’s urine and Broncho alveolar lavage culture grew Klebsiella Pneumonia and, as per sensitivity, same antibiotic was continued for the patient.
After 5th dose of IV antibiotics patient developed blisters filled with fluids (bullous) around the IV lines. On examining, the patient’s skin texture was very thin, pinkish to red in colour, so the rashes went unnoticed. IV line was checked for blockage and lines were clear. Dermatologist opinion sough, and initiated a treatment with Fucidin H cream for rashes. Patient was discharged in a stable condition.
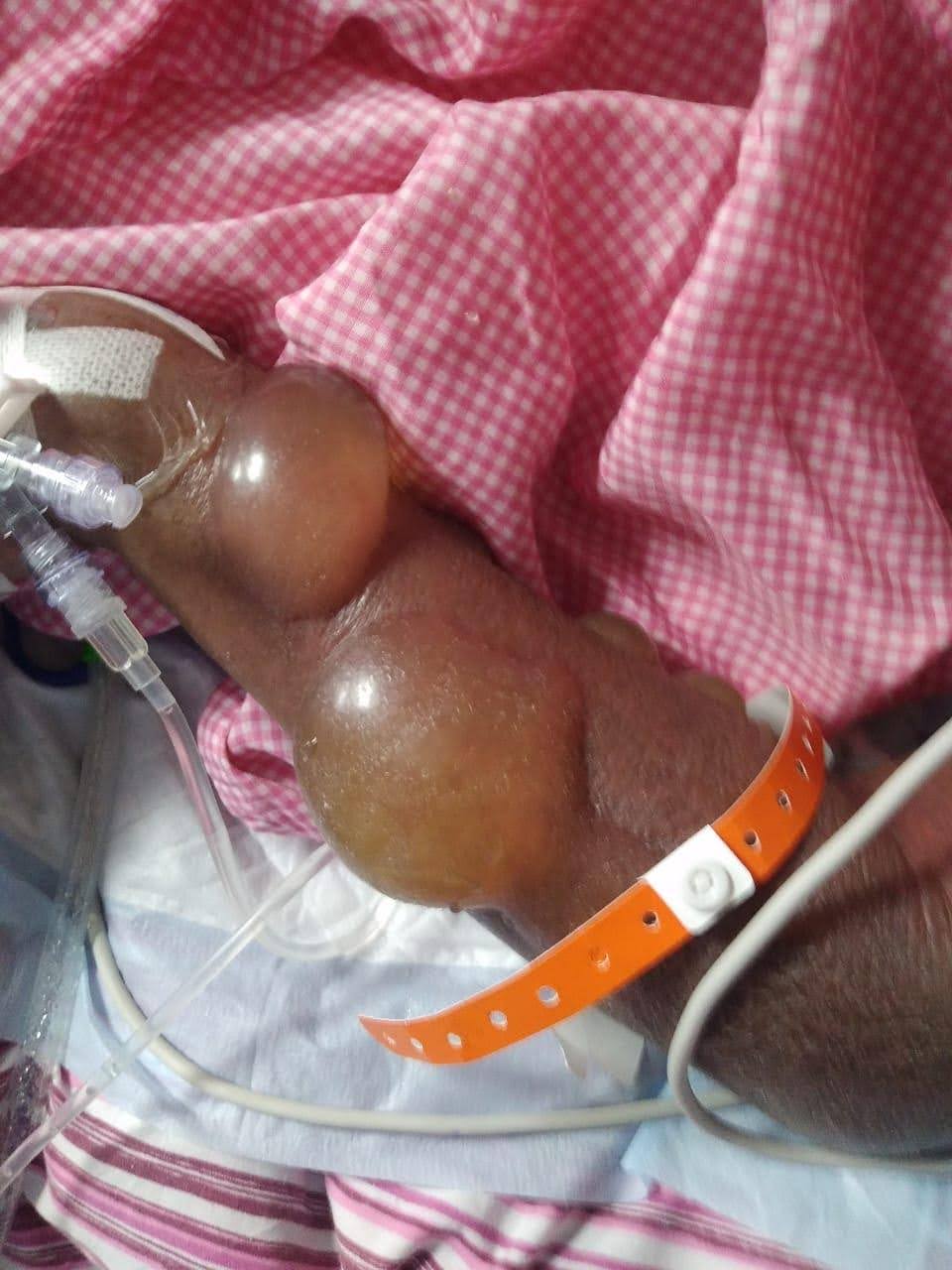
Fig (1): Represents the Fluid Filled blisters after the administration of Cefepime- Tazobactum.
Discussion
- The adverse reaction of fluid filled blisters, occurring following cefepime injection, is rare but is a documented side effect associated with cephalosporins. Cefepime, a fourth generation cephalosporins, has unique chemical structure compared to other cephalosporins. This may contribute to distinct adverse reactions. The exact mechanisms leading to these reactions are not fully understood. Hypersensitivity reactions, immune complex deposition, and direct toxic effects have been proposed to be the causes. Cross reactivity among cephalosporins varies between different generations and individual drugs. [1] Drug-associated Bullous Pemphigoid (DABP) is a term used to describe instances of BP demonstrating clinical, histological, or immunopathological features identical or similar to those of the idiopathic form of the disease. DABP may be associated with the systemic ingestion or topical application of particular drugs.[2]
- The pathogenesis of drug-induced bullous pemphigoid (DIBP) is complex, especially in the elderly taking multiple medications. The “two steps” theory suggests that interactions between structurally similar drugs trigger an immune response, leading to blistering. Pinpointing specific causative drugs is challenging due to polypharmacy. Comprehensive patient history is crucial for diagnosis, and ongoing research is needed for a better understanding of DIBP.[3]
Reference
- Romano A, Blanca M, Torres MJ, Bircher A, Aberer W, Brockow K, Pichler WJ, Demoly P; ENDA; EAACI. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004 Nov;59(11):1153-60.
- Vassileva S. Drug-Induced Pemphigoid:: Bullous and Cicatricial. Clinics in dermatology. 1998 May 1;16(3):379-87
- Sanderson BK, Naisbitt DJ. Drugs as Haptens, Antigens, and Immunogens. Drug Hypersensitivity. 2007:55.

K. Sathyapriya
Senior Clinical Pharmacist
